[English] 日本語
 Yorodumi
Yorodumi- EMDB-2149: Electron microscopy of Influenza hemagglutinin (Wisconsin/1/1966 ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-2149 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Electron microscopy of Influenza hemagglutinin (Wisconsin/1/1966 (H9N2)) in complex with neutralizing antibody (Fab CR9114) | |||||||||
 Map data Map data | Influenza hemagglutinin (Wisconsin/1/1966 (H9N2))), neutralizing antibody (Fab CR9114), negative stained, single particle analysis | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Influenza hemagglutinin (Wisconsin/1/1966 (H9N2))) / neutralizing antibody (Fab CR9114) / negative stained / single particle analysis | |||||||||
| Biological species |  Influenza A virus (A/turkey/Wisconsin/1/1966(H9N2)) / Influenza A virus (A/turkey/Wisconsin/1/1966(H9N2)) /  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / negative staining / Resolution: 14.8 Å | |||||||||
 Authors Authors | Khayat R / Meltagen Z / Ekiert DC / Dreyfus C / Wilson IA / Ward AB | |||||||||
 Citation Citation |  Journal: Science / Year: 2012 Journal: Science / Year: 2012Title: Highly conserved protective epitopes on influenza B viruses. Authors: Cyrille Dreyfus / Nick S Laursen / Ted Kwaks / David Zuijdgeest / Reza Khayat / Damian C Ekiert / Jeong Hyun Lee / Zoltan Metlagel / Miriam V Bujny / Mandy Jongeneelen / Remko van der Vlugt ...Authors: Cyrille Dreyfus / Nick S Laursen / Ted Kwaks / David Zuijdgeest / Reza Khayat / Damian C Ekiert / Jeong Hyun Lee / Zoltan Metlagel / Miriam V Bujny / Mandy Jongeneelen / Remko van der Vlugt / Mohammed Lamrani / Hans J W M Korse / Eric Geelen / Özcan Sahin / Martijn Sieuwerts / Just P J Brakenhoff / Ronald Vogels / Olive T W Li / Leo L M Poon / Malik Peiris / Wouter Koudstaal / Andrew B Ward / Ian A Wilson / Jaap Goudsmit / Robert H E Friesen /  Abstract: Identification of broadly neutralizing antibodies against influenza A viruses has raised hopes for the development of monoclonal antibody-based immunotherapy and "universal" vaccines for influenza. ...Identification of broadly neutralizing antibodies against influenza A viruses has raised hopes for the development of monoclonal antibody-based immunotherapy and "universal" vaccines for influenza. However, a substantial part of the annual flu burden is caused by two cocirculating, antigenically distinct lineages of influenza B viruses. Here, we report human monoclonal antibodies, CR8033, CR8071, and CR9114, that protect mice against lethal challenge from both lineages. Antibodies CR8033 and CR8071 recognize distinct conserved epitopes in the head region of the influenza B hemagglutinin (HA), whereas CR9114 binds a conserved epitope in the HA stem and protects against lethal challenge with influenza A and B viruses. These antibodies may inform on development of monoclonal antibody-based treatments and a universal flu vaccine for all influenza A and B viruses. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_2149.map.gz emd_2149.map.gz | 12.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-2149-v30.xml emd-2149-v30.xml emd-2149.xml emd-2149.xml | 13.3 KB 13.3 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_2149.png emd_2149.png | 32.7 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-2149 http://ftp.pdbj.org/pub/emdb/structures/EMD-2149 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-2149 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-2149 | HTTPS FTP |
-Related structure data
| Related structure data |  2143C  2144C  2145C  2146C  2147C  2148C  2150C  4fqhC  4fqiC  4fqjC  4fqkC  4fqlC  4fqmC  4fqvC  4fqyC C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_2149.map.gz / Format: CCP4 / Size: 15.3 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_2149.map.gz / Format: CCP4 / Size: 15.3 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Influenza hemagglutinin (Wisconsin/1/1966 (H9N2))), neutralizing antibody (Fab CR9114), negative stained, single particle analysis | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 2.18 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
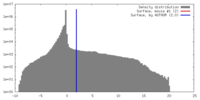
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Influenza hemagglutinin (Wisconsin/1/1966 (H9N2)) in complex with...
| Entire | Name: Influenza hemagglutinin (Wisconsin/1/1966 (H9N2)) in complex with neutralizing IgG antibody fragment(Fab CR9114) |
|---|---|
| Components |
|
-Supramolecule #1000: Influenza hemagglutinin (Wisconsin/1/1966 (H9N2)) in complex with...
| Supramolecule | Name: Influenza hemagglutinin (Wisconsin/1/1966 (H9N2)) in complex with neutralizing IgG antibody fragment(Fab CR9114) type: sample / ID: 1000 / Details: The sample was monodisperse / Oligomeric state: One hemagglutinin trimer binds 3 Fabs / Number unique components: 2 |
|---|---|
| Molecular weight | Experimental: 282 KDa / Theoretical: 282 KDa / Method: Amino acid sequence |
-Macromolecule #1: Influenza hemagglutinin (Wisconsin/1/1966 (H9N2)))
| Macromolecule | Name: Influenza hemagglutinin (Wisconsin/1/1966 (H9N2))) / type: protein_or_peptide / ID: 1 / Number of copies: 3 / Oligomeric state: Trimer / Recombinant expression: Yes |
|---|---|
| Source (natural) | Organism:  Influenza A virus (A/turkey/Wisconsin/1/1966(H9N2)) Influenza A virus (A/turkey/Wisconsin/1/1966(H9N2)) |
| Molecular weight | Experimental: 49 KDa / Theoretical: 49 KDa |
| Recombinant expression | Organism: Sf9 / Recombinant plasmid: pDCE198 |
-Macromolecule #2: IgG Antibody fragment (Fab CR9114)
| Macromolecule | Name: IgG Antibody fragment (Fab CR9114) / type: protein_or_peptide / ID: 2 / Number of copies: 3 / Oligomeric state: 3 monomers / Recombinant expression: Yes |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Experimental: 45 KDa / Theoretical: 45 KDa |
-Experimental details
-Structure determination
| Method | negative staining |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.1 mg/mL |
|---|---|
| Buffer | pH: 8 / Details: 20 mM HEPES 8.0, 50mM NaCl |
| Staining | Type: NEGATIVE Details: Grids with adsorbed protein floated on 2% w/v uranyl formate for 30 seconds. |
| Grid | Details: 400 mesh gold grid with thin carbon support, glow discharged in amylamine atmosphere |
| Vitrification | Cryogen name: NONE / Chamber humidity: 18 % / Instrument: OTHER |
- Electron microscopy
Electron microscopy
| Microscope | FEI TECNAI F20 |
|---|---|
| Alignment procedure | Legacy - Astigmatism: Objective lens astigmatism was corrected at 100,000 times magnification |
| Details | Weak beam illumination |
| Date | May 3, 2011 |
| Image recording | Category: CCD / Film or detector model: GATAN ULTRASCAN 4000 (4k x 4k) / Digitization - Sampling interval: 10.9 µm / Number real images: 165 / Average electron dose: 16 e/Å2 / Od range: 1.4 / Bits/pixel: 16 |
| Tilt angle min | 0 |
| Electron beam | Acceleration voltage: 120 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated magnification: 100000 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2 mm / Nominal defocus max: 1.2 µm / Nominal defocus min: 0.6 µm / Nominal magnification: 100000 |
| Sample stage | Specimen holder model: SIDE ENTRY, EUCENTRIC / Tilt angle max: 55 |
| Experimental equipment |  Model: Tecnai F20 / Image courtesy: FEI Company |
- Image processing
Image processing
| Details | Particles were selected using DoG Picker and processed using a combination of EMAN2 and Sparx. |
|---|---|
| CTF correction | Details: Each particle |
| Final reconstruction | Applied symmetry - Point group: C3 (3 fold cyclic) / Algorithm: OTHER / Resolution.type: BY AUTHOR / Resolution: 14.8 Å / Resolution method: FSC 0.5 CUT-OFF / Software - Name: EMAN2, and, Sparx / Number images used: 6629 |
-Atomic model buiding 1
| Initial model | PDB ID: |
|---|---|
| Software | Name: Molrep, Chimera |
| Details | Protocol: Rigid body. The hemagluttinin trimer was fit, then the Fabs were fit while imposing 3-fold non-cyrstallographic symmetry using Molrep. |
| Refinement | Space: REAL / Protocol: RIGID BODY FIT / Target criteria: Correlation coefficient |
-Atomic model buiding 2
| Initial model | PDB ID: |
|---|---|
| Software | Name: Molrep, Chimera |
| Details | Protocol: Rigid body. The hemagluttinin trimer was fit, then the Fabs were fit while imposing 3-fold non-cyrstallographic symmetry using Molrep. |
| Refinement | Space: REAL / Protocol: RIGID BODY FIT / Target criteria: Correlation coefficient |
 Movie
Movie Controller
Controller










 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)