+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-21434 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Bestrophin-2 (BEST2) calcium-unbound state 1 (EGTA only) | |||||||||||||||
 Map data Map data | Bestrophin-2 (BEST2) calcium-free state 1 (EGTA only) | |||||||||||||||
 Sample Sample |
| |||||||||||||||
 Keywords Keywords | Chloride Channel / MEMBRANE PROTEIN | |||||||||||||||
| Function / homology |  Function and homology information Function and homology informationStimuli-sensing channels / intracellularly ligand-gated monoatomic ion channel activity / ligand-gated monoatomic anion channel activity / bicarbonate channel activity / bicarbonate transport / ligand-gated monoatomic cation channel activity / chloride channel activity / chloride channel complex / basolateral plasma membrane / plasma membrane Similarity search - Function | |||||||||||||||
| Biological species |  | |||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.17 Å | |||||||||||||||
 Authors Authors | Owji AP / Zhao Q | |||||||||||||||
| Funding support |  United States, 4 items United States, 4 items
| |||||||||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2020 Journal: Nat Struct Mol Biol / Year: 2020Title: Structural and functional characterization of the bestrophin-2 anion channel. Authors: Aaron P Owji / Qingqing Zhao / Changyi Ji / Alec Kittredge / Austin Hopiavuori / Ziao Fu / Nancy Ward / Oliver B Clarke / Yin Shen / Yu Zhang / Wayne A Hendrickson / Tingting Yang /   Abstract: The bestrophin family of calcium (Ca)-activated chloride (Cl) channels, which mediate the influx and efflux of monovalent anions in response to the levels of intracellular Ca, comprises four members ...The bestrophin family of calcium (Ca)-activated chloride (Cl) channels, which mediate the influx and efflux of monovalent anions in response to the levels of intracellular Ca, comprises four members in mammals (bestrophin 1-4). Here we report cryo-EM structures of bovine bestrophin-2 (bBest2) bound and unbound by Ca at 2.4- and 2.2-Å resolution, respectively. The bBest2 structure highlights four previously underappreciated pore-lining residues specifically conserved in Best2 but not in Best1, illustrating the differences between these paralogs. Structure-inspired electrophysiological analysis reveals that, although the channel is sensitive to Ca, it has substantial Ca-independent activity for Cl, reflecting the opening at the cytoplasmic restriction of the ion conducting pathway even when Ca is absent. Moreover, the ion selectivity of bBest2 is controlled by multiple residues, including those involved in gating. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_21434.map.gz emd_21434.map.gz | 134.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-21434-v30.xml emd-21434-v30.xml emd-21434.xml emd-21434.xml | 17.6 KB 17.6 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_21434.png emd_21434.png | 68.6 KB | ||
| Filedesc metadata |  emd-21434.cif.gz emd-21434.cif.gz | 5.6 KB | ||
| Others |  emd_21434_additional.map.gz emd_21434_additional.map.gz emd_21434_half_map_1.map.gz emd_21434_half_map_1.map.gz emd_21434_half_map_2.map.gz emd_21434_half_map_2.map.gz | 33.1 MB 59.3 MB 59.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-21434 http://ftp.pdbj.org/pub/emdb/structures/EMD-21434 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-21434 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-21434 | HTTPS FTP |
-Related structure data
| Related structure data |  6vx9MC  6vx5C  6vx6C  6vx7C  6vx8C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_21434.map.gz / Format: CCP4 / Size: 144.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_21434.map.gz / Format: CCP4 / Size: 144.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Bestrophin-2 (BEST2) calcium-free state 1 (EGTA only) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.35128 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
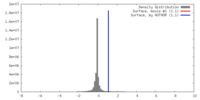
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Additional map: Additional map
| File | emd_21434_additional.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Additional map | ||||||||||||
| Projections & Slices |
| ||||||||||||
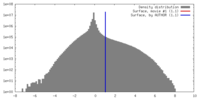
| Density Histograms |
-Half map: half-volume 1
| File | emd_21434_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half-volume 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
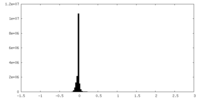
| Density Histograms |
-Half map: half-volume 2
| File | emd_21434_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half-volume 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
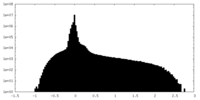
| Density Histograms |
- Sample components
Sample components
-Entire : bestrophin-2 (BEST2) calcium-free state 1 (EGTA only)
| Entire | Name: bestrophin-2 (BEST2) calcium-free state 1 (EGTA only) |
|---|---|
| Components |
|
-Supramolecule #1: bestrophin-2 (BEST2) calcium-free state 1 (EGTA only)
| Supramolecule | Name: bestrophin-2 (BEST2) calcium-free state 1 (EGTA only) / type: cell / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Bestrophin
| Macromolecule | Name: Bestrophin / type: protein_or_peptide / ID: 1 / Number of copies: 5 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 47.424754 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MTVTYTARVA KARFGGFSKL LLLWRGSIYK LLWRELLCFL GLFMALSAAY RFVLTEEQKR YFEKLVLYCD RYASLIPVSF VLGFYVTLV VHRWWNQYLS MPLTDALMCV VVGTVHGHDE RGRLYRRTLM RYAGLSGVLI LRSVSTAVFK RFPTIDHVVE A GFMTREER ...String: MTVTYTARVA KARFGGFSKL LLLWRGSIYK LLWRELLCFL GLFMALSAAY RFVLTEEQKR YFEKLVLYCD RYASLIPVSF VLGFYVTLV VHRWWNQYLS MPLTDALMCV VVGTVHGHDE RGRLYRRTLM RYAGLSGVLI LRSVSTAVFK RFPTIDHVVE A GFMTREER KKFENLNSSY NKYWVPCVWF CNLAAQARRE GRIRDNGAFK LLLEELNVFR SKCGMLFHYD WISVPLVYTQ VV TIAVYSY FLACLIGRQF LDPAQGYKDH DLDLCVPIFT LLQFFFYAGW LKVAEQLINP FGEDDDDFET NFLIDRCFQV SML AVDEMY DDLAMLEKDL YWDAAEARAP YTAATAFLMQ QPSFQGSTFD ITLAKEDMQF QRQDGLEAPL NEAHGDFLQR LLPV GTGMG TGGLL UniProtKB: Bestrophin-2 |
-Macromolecule #2: CHLORIDE ION
| Macromolecule | Name: CHLORIDE ION / type: ligand / ID: 2 / Number of copies: 1 / Formula: CL |
|---|---|
| Molecular weight | Theoretical: 35.453 Da |
-Macromolecule #3: water
| Macromolecule | Name: water / type: ligand / ID: 3 / Number of copies: 455 / Formula: HOH |
|---|---|
| Molecular weight | Theoretical: 18.015 Da |
| Chemical component information |  ChemComp-HOH: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.8 mg/mL |
|---|---|
| Buffer | pH: 7.8 |
| Vitrification | Cryogen name: ETHANE / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Average electron dose: 69.67 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: NONE |
|---|---|
| Final reconstruction | Applied symmetry - Point group: C5 (5 fold cyclic) / Resolution.type: BY AUTHOR / Resolution: 2.17 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: cryoSPARC (ver. 2.13.2) / Number images used: 280524 |
| Initial angle assignment | Type: ANGULAR RECONSTITUTION |
| Final angle assignment | Type: ANGULAR RECONSTITUTION |
 Movie
Movie Controller
Controller












 Z (Sec.)
Z (Sec.) X (Row.)
X (Row.) Y (Col.)
Y (Col.)