[English] 日本語
 Yorodumi
Yorodumi- EMDB-21023: Single-particle cryo-EM reconstruction of rabbit muscle aldolase ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-21023 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Single-particle cryo-EM reconstruction of rabbit muscle aldolase using 200 keV | |||||||||
 Map data Map data | Single-particle cryo-EM reconstruction of rabbit muscle aldolase | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | homo-4-mer / lyase / D-fructose / glycolysis | |||||||||
| Function / homology |  Function and homology information Function and homology informationnegative regulation of Arp2/3 complex-mediated actin nucleation / fructose-bisphosphate aldolase / fructose-bisphosphate aldolase activity / M band / I band / glycolytic process / protein homotetramerization / positive regulation of cell migration Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.13 Å | |||||||||
 Authors Authors | Wu M / Lander GC | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: J Struct Biol X / Year: 2020 Journal: J Struct Biol X / Year: 2020Title: Sub-2 Angstrom resolution structure determination using single-particle cryo-EM at 200 keV. Authors: Mengyu Wu / Gabriel C Lander / Mark A Herzik /  Abstract: Although the advent of direct electron detectors (DEDs) and software developments have enabled the routine use of single-particle cryogenic electron microscopy (cryo-EM) for structure determination ...Although the advent of direct electron detectors (DEDs) and software developments have enabled the routine use of single-particle cryogenic electron microscopy (cryo-EM) for structure determination of well-behaved specimens to high-resolution, there nonetheless remains a discrepancy between the resolutions attained for biological specimens and the information limits of modern transmission electron microscopes (TEMs). Instruments operating at 300 kV equipped with DEDs are the current paradigm for high-resolution single-particle cryo-EM, while 200 kV TEMs remain comparatively underutilized for purposes beyond sample screening. Here, we expand upon our prior work and demonstrate that one such 200 kV microscope, the Talos Arctica, equipped with a K2 DED is capable of determining structures of macromolecules to as high as ∼1.7 Å resolution. At this resolution, ordered water molecules are readily assigned and holes in aromatic residues can be clearly distinguished in the reconstructions. This work emphasizes the utility of 200 kV electrons for high-resolution single-particle cryo-EM and applications such as structure-based drug design. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_21023.map.gz emd_21023.map.gz | 59.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-21023-v30.xml emd-21023-v30.xml emd-21023.xml emd-21023.xml | 21.1 KB 21.1 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_21023_fsc.xml emd_21023_fsc.xml | 18.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_21023.png emd_21023.png | 231.9 KB | ||
| Masks |  emd_21023_msk_1.map emd_21023_msk_1.map | 512 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-21023.cif.gz emd-21023.cif.gz | 6.6 KB | ||
| Others |  emd_21023_additional.map.gz emd_21023_additional.map.gz emd_21023_half_map_1.map.gz emd_21023_half_map_1.map.gz emd_21023_half_map_2.map.gz emd_21023_half_map_2.map.gz | 409.7 MB 410.9 MB 411.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-21023 http://ftp.pdbj.org/pub/emdb/structures/EMD-21023 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-21023 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-21023 | HTTPS FTP |
-Related structure data
| Related structure data |  6v20MC  6v21C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | |
| EM raw data |  EMPIAR-10338 (Title: Rabbit muscle aldolase movies obtained using a Talos Arctica (200 kV) equipped with a K2 EMPIAR-10338 (Title: Rabbit muscle aldolase movies obtained using a Talos Arctica (200 kV) equipped with a K2Data size: 543.3 Data #1: Rabbit muscle aldolase movies obtained using a Talos Arctica (200 kV) equipped with a K2 [micrographs - multiframe]) |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_21023.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_21023.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Single-particle cryo-EM reconstruction of rabbit muscle aldolase | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.562 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_21023_msk_1.map emd_21023_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
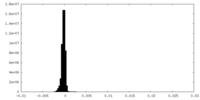
| Density Histograms |
-Additional map: Unsharpened map
| File | emd_21023_additional.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Unsharpened map | ||||||||||||
| Projections & Slices |
| ||||||||||||
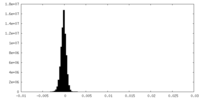
| Density Histograms |
-Half map: Odd half map
| File | emd_21023_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Odd half map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Even half map
| File | emd_21023_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Even half map | ||||||||||||
| Projections & Slices |
| ||||||||||||
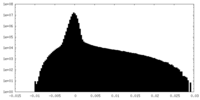
| Density Histograms |
- Sample components
Sample components
-Entire : Aldolase from rabbit muscle
| Entire | Name: Aldolase from rabbit muscle |
|---|---|
| Components |
|
-Supramolecule #1: Aldolase from rabbit muscle
| Supramolecule | Name: Aldolase from rabbit muscle / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 Details: Lyophilized rabbit muscle aldolase purchased from Sigma Aldrich was further purified to homogeneity. |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 150 KDa |
-Macromolecule #1: Fructose-bisphosphate aldolase A
| Macromolecule | Name: Fructose-bisphosphate aldolase A / type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO / EC number: fructose-bisphosphate aldolase |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 37.302602 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: HSHPALTPEQ KKELSDIAHR IVAPGKGILA ADESTGSIAK RLQSIGTENT EENRRFYRQL LLTADDRVNP CIGGVILFHE TLYQKADDG RPFPQVIKSK GGVVGIKVDK GVVPLAGTNG ETTTQGLDGL SERCAQYKKD GADFAKWRCV LKIGEHTPSA L AIMENANV ...String: HSHPALTPEQ KKELSDIAHR IVAPGKGILA ADESTGSIAK RLQSIGTENT EENRRFYRQL LLTADDRVNP CIGGVILFHE TLYQKADDG RPFPQVIKSK GGVVGIKVDK GVVPLAGTNG ETTTQGLDGL SERCAQYKKD GADFAKWRCV LKIGEHTPSA L AIMENANV LARYASICQQ NGIVPIVEPE ILPDGDHDLK RCQYVTEKVL AAVYKALSDH HIYLEGTLLK PNMVTPGHAC TQ KYSHEEI AMATVTALRR TVPPAVTGVT FLSGGQSEEE ASINLNAINK CPLLKPWALT FSYGRALQAS ALKAWGGKKE NLK AAQEEY VKRALANSLA CQGKYTP UniProtKB: Fructose-bisphosphate aldolase A |
-Macromolecule #2: water
| Macromolecule | Name: water / type: ligand / ID: 2 / Number of copies: 328 / Formula: HOH |
|---|---|
| Molecular weight | Theoretical: 18.015 Da |
| Chemical component information |  ChemComp-HOH: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1.6 mg/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.5 Component:
| |||||||||
| Grid | Model: UltrAuFoil / Material: GOLD / Mesh: 300 / Pretreatment - Type: PLASMA CLEANING / Pretreatment - Time: 7 sec. / Pretreatment - Atmosphere: OTHER / Details: 15 Watts | |||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277.15 K / Instrument: HOMEMADE PLUNGER Details: 3 uL of sample/grid was manually blotted for 4 seconds prior to immediate plunge-freezing in liquid nitrogen-cooled ethane.. | |||||||||
| Details | Reconstituted from lyophilized form (Sigma Aldrich) |
- Electron microscopy
Electron microscopy
| Microscope | FEI TALOS ARCTICA |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Digitization - Dimensions - Width: 3710 pixel / Digitization - Dimensions - Height: 3838 pixel / Digitization - Frames/image: 1-44 / Number grids imaged: 1 / Number real images: 3905 / Average exposure time: 11.0 sec. / Average electron dose: 67.0 e/Å2 Details: Images were collected using stage position navigation to target exposure. |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 1.8 µm / Nominal defocus min: 0.3 µm / Nominal magnification: 73000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Talos Arctica / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model | PDB ID: Chain - Source name: PDB / Chain - Initial model type: experimental model |
|---|---|
| Details | Starting model was generated by stripping PDB entry 5VY5 of all ligands and alternate conformations, then refining into the EM density using imposed symmetry while adjusting weighting/scoring according to estimated map resolution. The top 10 generated models (ranked based on quality metrics) were real-space refined using Phenix software. |
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT / Overall B value: 30 |
| Output model |  PDB-6v20: |
 Movie
Movie Controller
Controller













 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)