+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-2084 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structures from COPI-coated vesicles: triad | |||||||||
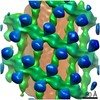
 Map data Map data | Reconstruction of a triad | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | COPI-coated vesicles / subtomogram averaging / membrane trafficking / protein coat / coatomer / COPI | |||||||||
| Biological species | unidentified (others) /   | |||||||||
| Method | subtomogram averaging / cryo EM / Resolution: 26.0 Å | |||||||||
 Authors Authors | Faini M / Prinz S / Beck R / Schorb M / Riches JD / Bacia K / Brugger B / Wieland FT / Briggs JAG | |||||||||
 Citation Citation |  Journal: Science / Year: 2012 Journal: Science / Year: 2012Title: The structures of COPI-coated vesicles reveal alternate coatomer conformations and interactions. Authors: Marco Faini / Simone Prinz / Rainer Beck / Martin Schorb / James D Riches / Kirsten Bacia / Britta Brügger / Felix T Wieland / John A G Briggs /  Abstract: Transport between compartments of eukaryotic cells is mediated by coated vesicles. The archetypal protein coats COPI, COPII, and clathrin are conserved from yeast to human. Structural studies of ...Transport between compartments of eukaryotic cells is mediated by coated vesicles. The archetypal protein coats COPI, COPII, and clathrin are conserved from yeast to human. Structural studies of COPII and clathrin coats assembled in vitro without membranes suggest that coat components assemble regular cages with the same set of interactions between components. Detailed three-dimensional structures of coated membrane vesicles have not been obtained. Here, we solved the structures of individual COPI-coated membrane vesicles by cryoelectron tomography and subtomogram averaging of in vitro reconstituted budding reactions. The coat protein complex, coatomer, was observed to adopt alternative conformations to change the number of other coatomers with which it interacts and to form vesicles with variable sizes and shapes. This represents a fundamentally different basis for vesicle coat assembly. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_2084.map.gz emd_2084.map.gz | 3.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-2084-v30.xml emd-2084-v30.xml emd-2084.xml emd-2084.xml | 10.8 KB 10.8 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_2084.tif emd_2084.tif | 176.9 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-2084 http://ftp.pdbj.org/pub/emdb/structures/EMD-2084 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-2084 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-2084 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_2084.map.gz / Format: CCP4 / Size: 5.2 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_2084.map.gz / Format: CCP4 / Size: 5.2 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Reconstruction of a triad | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 4.94 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : triad
| Entire | Name: triad |
|---|---|
| Components |
|
-Supramolecule #1000: triad
| Supramolecule | Name: triad / type: sample / ID: 1000 / Number unique components: 3 |
|---|
-Supramolecule #1: liposome membrane
| Supramolecule | Name: liposome membrane / type: organelle_or_cellular_component / ID: 1 / Recombinant expression: No / Database: NCBI |
|---|---|
| Source (natural) | Organism: unidentified (others) |
-Macromolecule #1: Coatomer complex
| Macromolecule | Name: Coatomer complex / type: protein_or_peptide / ID: 1 / Name.synonym: COPI / Number of copies: 3 / Recombinant expression: Yes |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 560 KDa |
| Recombinant expression | Organism:  unidentified baculovirus unidentified baculovirus |
-Macromolecule #2: Arf1
| Macromolecule | Name: Arf1 / type: protein_or_peptide / ID: 2 / Recombinant expression: Yes |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 20 KDa |
| Recombinant expression | Organism:  |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | subtomogram averaging |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 / Details: 50mM Hepes , 50 mM KOAc, 1 mM MgCl2 |
|---|---|
| Grid | Details: 300 mesh copper grid with holey carbon support, glow discharged |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 90 K / Instrument: OTHER / Method: Hand plunging |
- Electron microscopy
Electron microscopy
| Microscope | FEI POLARA 300 |
|---|---|
| Temperature | Min: 90 K |
| Specialist optics | Energy filter - Name: Gatan GIF 2000 |
| Date | May 6, 2011 |
| Image recording | Category: CCD / Film or detector model: GENERIC CCD / Average electron dose: 60 e/Å2 / Bits/pixel: 14 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.0 mm / Nominal defocus max: 4.3 µm / Nominal defocus min: 2.3 µm / Nominal magnification: 27500 |
| Sample stage | Specimen holder model: GATAN LIQUID NITROGEN / Tilt series - Axis1 - Min angle: -60 ° / Tilt series - Axis1 - Max angle: 60 ° |
| Experimental equipment |  Model: Tecnai Polara / Image courtesy: FEI Company |
- Image processing
Image processing
| Details | CTF correction was performed. Average number of tilts used in the 3D reconstructions: 41. Average tomographic tilt angle increment: 3. |
|---|---|
| Final reconstruction | Algorithm: OTHER / Resolution.type: BY AUTHOR / Resolution: 26.0 Å / Resolution method: OTHER / Software - Name: IMOD, TOM, package, Matlab |
| CTF correction | Details: CTF multiplication |
 Movie
Movie Controller
Controller













 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)





















