[English] 日本語
 Yorodumi
Yorodumi- EMDB-20731: Full length Glycine receptor reconstituted in lipid nanodisc in G... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-20731 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Full length Glycine receptor reconstituted in lipid nanodisc in Gly/PTX-bound open/blocked conformation | |||||||||
 Map data Map data | Refine3D Map generated during 3D autorefinement in Relion3.1 beta | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Ions Ligands Receptors / Glycine receptor Recombinant Proteins Glycine / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationNeurotransmitter receptors and postsynaptic signal transmission / extracellularly glycine-gated ion channel activity / extracellularly glycine-gated chloride channel activity / transmitter-gated monoatomic ion channel activity / regulation of neuron differentiation / ligand-gated monoatomic ion channel activity / glycine binding / cellular response to zinc ion / cellular response to ethanol / response to amino acid ...Neurotransmitter receptors and postsynaptic signal transmission / extracellularly glycine-gated ion channel activity / extracellularly glycine-gated chloride channel activity / transmitter-gated monoatomic ion channel activity / regulation of neuron differentiation / ligand-gated monoatomic ion channel activity / glycine binding / cellular response to zinc ion / cellular response to ethanol / response to amino acid / chloride channel complex / monoatomic ion transport / chloride transmembrane transport / central nervous system development / cellular response to amino acid stimulus / transmembrane signaling receptor activity / perikaryon / postsynaptic membrane / dendrite / zinc ion binding / membrane / plasma membrane Similarity search - Function | |||||||||
| Biological species |  | |||||||||
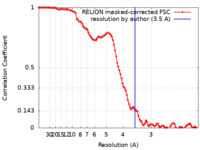
| Method | single particle reconstruction / cryo EM / Resolution: 3.5 Å | |||||||||
 Authors Authors | Kumar A / Basak S / Chakrapani S | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2015 Journal: Nature / Year: 2015Title: Glycine receptor mechanism elucidated by electron cryo-microscopy. Authors: Juan Du / Wei Lü / Shenping Wu / Yifan Cheng / Eric Gouaux /  Abstract: The strychnine-sensitive glycine receptor (GlyR) mediates inhibitory synaptic transmission in the spinal cord and brainstem and is linked to neurological disorders, including autism and hyperekplexia. ...The strychnine-sensitive glycine receptor (GlyR) mediates inhibitory synaptic transmission in the spinal cord and brainstem and is linked to neurological disorders, including autism and hyperekplexia. Understanding of molecular mechanisms and pharmacology of glycine receptors has been hindered by a lack of high-resolution structures. Here we report electron cryo-microscopy structures of the zebrafish α1 GlyR with strychnine, glycine, or glycine and ivermectin (glycine/ivermectin). Strychnine arrests the receptor in an antagonist-bound closed ion channel state, glycine stabilizes the receptor in an agonist-bound open channel state, and the glycine/ivermectin complex adopts a potentially desensitized or partially open state. Relative to the glycine-bound state, strychnine expands the agonist-binding pocket via outward movement of the C loop, promotes rearrangement of the extracellular and transmembrane domain 'wrist' interface, and leads to rotation of the transmembrane domain towards the pore axis, occluding the ion conduction pathway. These structures illuminate the GlyR mechanism and define a rubric to interpret structures of Cys-loop receptors. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_20731.map.gz emd_20731.map.gz | 80.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-20731-v30.xml emd-20731-v30.xml emd-20731.xml emd-20731.xml | 21.8 KB 21.8 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_20731_fsc.xml emd_20731_fsc.xml | 10.7 KB | Display |  FSC data file FSC data file |
| Images |  emd_20731.png emd_20731.png | 49.2 KB | ||
| Masks |  emd_20731_msk_1.map emd_20731_msk_1.map | 103 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-20731.cif.gz emd-20731.cif.gz | 6.6 KB | ||
| Others |  emd_20731_additional.map.gz emd_20731_additional.map.gz emd_20731_half_map_1.map.gz emd_20731_half_map_1.map.gz emd_20731_half_map_2.map.gz emd_20731_half_map_2.map.gz | 95.8 MB 80.8 MB 80.8 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-20731 http://ftp.pdbj.org/pub/emdb/structures/EMD-20731 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-20731 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-20731 | HTTPS FTP |
-Validation report
| Summary document |  emd_20731_validation.pdf.gz emd_20731_validation.pdf.gz | 958.5 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_20731_full_validation.pdf.gz emd_20731_full_validation.pdf.gz | 958 KB | Display | |
| Data in XML |  emd_20731_validation.xml.gz emd_20731_validation.xml.gz | 17.9 KB | Display | |
| Data in CIF |  emd_20731_validation.cif.gz emd_20731_validation.cif.gz | 23.5 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-20731 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-20731 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-20731 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-20731 | HTTPS FTP |
-Related structure data
| Related structure data |  6ud3MC  6ubsC  6ubtC  6vm0C  6vm2C  6vm3C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_20731.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_20731.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Refine3D Map generated during 3D autorefinement in Relion3.1 beta | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.06 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_20731_msk_1.map emd_20731_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Postprocessmap generated using relion-postprocess masking out nanodisc belt...
| File | emd_20731_additional.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Postprocessmap generated using relion-postprocess masking out nanodisc belt | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Unfiltered maps reconstructed independently each using half of...
| File | emd_20731_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Unfiltered maps reconstructed independently each using half of the experimental data | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Unfiltered maps reconstructed independently each using half of...
| File | emd_20731_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Unfiltered maps reconstructed independently each using half of the experimental data | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Glycine receptor subunit alpha Z1
| Entire | Name: Glycine receptor subunit alpha Z1 |
|---|---|
| Components |
|
-Supramolecule #1: Glycine receptor subunit alpha Z1
| Supramolecule | Name: Glycine receptor subunit alpha Z1 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 250 kDa/nm |
-Macromolecule #1: Glycine receptor subunit alphaZ1
| Macromolecule | Name: Glycine receptor subunit alphaZ1 / type: protein_or_peptide / ID: 1 / Number of copies: 5 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 52.594648 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MFALGIYLWE TIVFFSLAAS QQAAARKAAS PMPPSEFLDK LMGKVSGYDA RIRPNFKGPP VNVTCNIFIN SFGSIAETTM DYRVNIFLR QQWNDPRLAY SEYPDDSLDL DPSMLDSIWK PDLFFANEKG ANFHEVTTDN KLLRISKNGN VLYSIRITLV L ACPMDLKN ...String: MFALGIYLWE TIVFFSLAAS QQAAARKAAS PMPPSEFLDK LMGKVSGYDA RIRPNFKGPP VNVTCNIFIN SFGSIAETTM DYRVNIFLR QQWNDPRLAY SEYPDDSLDL DPSMLDSIWK PDLFFANEKG ANFHEVTTDN KLLRISKNGN VLYSIRITLV L ACPMDLKN FPMDVQTCIM QLESFGYTMN DLIFEWDEKG AVQVADGLTL PQFILKEEKD LRYCTKHYNT GKFTCIEARF HL ERQMGYY LIQMYIPSLL IVILSWVSFW INMDAAPARV GLGITTVLTM TTQSSGSRAS LPKVSYVKAI DIWMAVCLLF VFS ALLEYA AVNFIARQHK ELLRFQRRRR HLKEDEAGDG RFSFAAYGMG PACLQAKDGM AIKGNNNNAP TSTNPPEKTV EEMR KLFIS RAKRIDTVSR VAFPLVFLIF NIFYWITYKI IRSEDIHKQG LVPRGSHHHH HHHH UniProtKB: Glycine receptor subunit alphaZ1 |
-Macromolecule #2: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 2 / Number of copies: 5 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Macromolecule #3: GLYCINE
| Macromolecule | Name: GLYCINE / type: ligand / ID: 3 / Number of copies: 5 / Formula: GLY |
|---|---|
| Molecular weight | Theoretical: 75.067 Da |
| Chemical component information |  ChemComp-GLY: |
-Macromolecule #4: (1aR,2aR,3S,6R,6aS,8aS,8bR,9R)-2a-hydroxy-8b-methyl-9-(prop-1-en-...
| Macromolecule | Name: (1aR,2aR,3S,6R,6aS,8aS,8bR,9R)-2a-hydroxy-8b-methyl-9-(prop-1-en-2-yl)hexahydro-3,6-methano-1,5,7-trioxacyclopenta[ij]c yclopropa[a]azulene-4,8(3H)-dione type: ligand / ID: 4 / Number of copies: 1 / Formula: RI5 |
|---|---|
| Molecular weight | Theoretical: 292.284 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.1 mg/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
| |||||||||
| Grid | Details: unspecified | |||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 4 K / Instrument: FEI VITROBOT MARK IV Details: 3.5 ul of 0.1 mg/ml protein solution was applied on a grid in the Vitrobot MkIV chamber set to 100% RH at 4 degC for 30s and then blotted for 2 s and plunged. | |||||||||
| Details | Full length Zebrafish GlyR alpha1 homopentamer reconsituted in Nanodisc |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Detector mode: SUPER-RESOLUTION / Number grids imaged: 4 / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm |
| Sample stage | Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller


















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)