[English] 日本語
 Yorodumi
Yorodumi- EMDB-19838: HSV-1 DNA polymerase-processivity factor complex in exonuclease s... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | HSV-1 DNA polymerase-processivity factor complex in exonuclease state active site with 1-bp DNA mismatch | |||||||||
 Map data Map data | Locally filtered local refinement map from cryoSPARC | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | DNA / Polymerase / Complex / TRANSFERASE | |||||||||
| Function / homology |  Function and homology information Function and homology informationDNA polymerase activity / DNA polymerase complex / bidirectional double-stranded viral DNA replication / 5'-3' exonuclease activity / ribonuclease H / DNA-templated DNA replication / RNA-DNA hybrid ribonuclease activity / DNA-directed DNA polymerase / DNA-directed DNA polymerase activity / nucleotide binding ...DNA polymerase activity / DNA polymerase complex / bidirectional double-stranded viral DNA replication / 5'-3' exonuclease activity / ribonuclease H / DNA-templated DNA replication / RNA-DNA hybrid ribonuclease activity / DNA-directed DNA polymerase / DNA-directed DNA polymerase activity / nucleotide binding / host cell nucleus / DNA binding Similarity search - Function | |||||||||
| Biological species |   Human alphaherpesvirus 1 strain KOS / synthetic construct (others) Human alphaherpesvirus 1 strain KOS / synthetic construct (others) | |||||||||
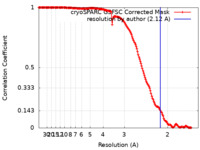
| Method | single particle reconstruction / cryo EM / Resolution: 2.12 Å | |||||||||
 Authors Authors | Gustavsson E / Grunewald K / Elias P / Hallberg BM | |||||||||
| Funding support |  Sweden, Sweden,  Germany, 2 items Germany, 2 items
| |||||||||
 Citation Citation |  Journal: Nucleic Acids Res / Year: 2024 Journal: Nucleic Acids Res / Year: 2024Title: Dynamics of the Herpes simplex virus DNA polymerase holoenzyme during DNA synthesis and proof-reading revealed by Cryo-EM. Authors: Emil Gustavsson / Kay Grünewald / Per Elias / B Martin Hällberg /   Abstract: Herpes simplex virus 1 (HSV-1), a double-stranded DNA virus, replicates using seven essential proteins encoded by its genome. Among these, the UL30 DNA polymerase, complexed with the UL42 ...Herpes simplex virus 1 (HSV-1), a double-stranded DNA virus, replicates using seven essential proteins encoded by its genome. Among these, the UL30 DNA polymerase, complexed with the UL42 processivity factor, orchestrates leading and lagging strand replication of the 152 kb viral genome. UL30 polymerase is a prime target for antiviral therapy, and resistance to current drugs can arise in immunocompromised individuals. Using electron cryo-microscopy (cryo-EM), we unveil the dynamic changes of the UL30/UL42 complex with DNA in three distinct states. First, a pre-translocation state with an open fingers domain ready for nucleotide incorporation. Second, a halted elongation state where the fingers close, trapping dATP in the dNTP pocket. Third, a DNA-editing state involving significant conformational changes to allow DNA realignment for exonuclease activity. Additionally, the flexible UL30 C-terminal domain interacts with UL42, forming an extended positively charged surface binding to DNA, thereby enhancing processive synthesis. These findings highlight substantial structural shifts in the polymerase and its DNA interactions during replication, offering insights for future antiviral drug development. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_19838.map.gz emd_19838.map.gz | 1.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-19838-v30.xml emd-19838-v30.xml emd-19838.xml emd-19838.xml | 23.9 KB 23.9 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_19838_fsc.xml emd_19838_fsc.xml | 15.7 KB | Display |  FSC data file FSC data file |
| Images |  emd_19838.png emd_19838.png | 69 KB | ||
| Masks |  emd_19838_msk_1.map emd_19838_msk_1.map | 421.9 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-19838.cif.gz emd-19838.cif.gz | 7.3 KB | ||
| Others |  emd_19838_additional_1.map.gz emd_19838_additional_1.map.gz emd_19838_half_map_1.map.gz emd_19838_half_map_1.map.gz emd_19838_half_map_2.map.gz emd_19838_half_map_2.map.gz | 212.5 MB 391.9 MB 391.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-19838 http://ftp.pdbj.org/pub/emdb/structures/EMD-19838 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-19838 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-19838 | HTTPS FTP |
-Related structure data
| Related structure data |  9enqMC  8oj6C  8oj7C  8ojaC  8ojbC  8ojcC  8ojdC  9enpC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_19838.map.gz / Format: CCP4 / Size: 421.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_19838.map.gz / Format: CCP4 / Size: 421.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Locally filtered local refinement map from cryoSPARC | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.8 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_19838_msk_1.map emd_19838_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
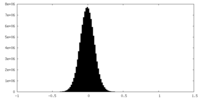
| Density Histograms |
-Additional map: Local refinement map from cryoSPARC
| File | emd_19838_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Local refinement map from cryoSPARC | ||||||||||||
| Projections & Slices |
| ||||||||||||
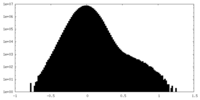
| Density Histograms |
-Half map: Local refinement halfmap A
| File | emd_19838_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Local refinement halfmap A | ||||||||||||
| Projections & Slices |
| ||||||||||||
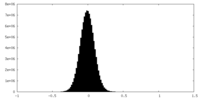
| Density Histograms |
-Half map: Local refinement halfmap B
| File | emd_19838_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Local refinement halfmap B | ||||||||||||
| Projections & Slices |
| ||||||||||||
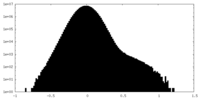
| Density Histograms |
- Sample components
Sample components
-Entire : HSV-1 DNA polymerase-processivity factor complex in exonuclease s...
| Entire | Name: HSV-1 DNA polymerase-processivity factor complex in exonuclease state with 1-bp DNA mismatch |
|---|---|
| Components |
|
-Supramolecule #1: HSV-1 DNA polymerase-processivity factor complex in exonuclease s...
| Supramolecule | Name: HSV-1 DNA polymerase-processivity factor complex in exonuclease state with 1-bp DNA mismatch type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#3 |
|---|
-Supramolecule #2: DNA polymerase-processivity factor
| Supramolecule | Name: DNA polymerase-processivity factor / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:   Human alphaherpesvirus 1 strain KOS Human alphaherpesvirus 1 strain KOS |
-Supramolecule #3: DNA
| Supramolecule | Name: DNA / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #2-#3 |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
-Macromolecule #1: DNA polymerase catalytic subunit
| Macromolecule | Name: DNA polymerase catalytic subunit / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO / EC number: DNA-directed DNA polymerase |
|---|---|
| Source (natural) | Organism:   Human alphaherpesvirus 1 strain KOS Human alphaherpesvirus 1 strain KOS |
| Molecular weight | Theoretical: 136.683844 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MFSGGGGPLS PGGKSAARAA SGFFAPAGPR GASRGPPPCL RQNFYNPYLA PVGTQQKPTG PTQRHTYYSE CDEFRFIAPR VLDEDAPPE KRAGVHDGHL KRAPKVYCGG DERDVLRVGS GGFWPRRSRL WGGVDHAPAG FNPTVTVFHV YDILENVEHA Y GMRAAQFH ...String: MFSGGGGPLS PGGKSAARAA SGFFAPAGPR GASRGPPPCL RQNFYNPYLA PVGTQQKPTG PTQRHTYYSE CDEFRFIAPR VLDEDAPPE KRAGVHDGHL KRAPKVYCGG DERDVLRVGS GGFWPRRSRL WGGVDHAPAG FNPTVTVFHV YDILENVEHA Y GMRAAQFH ARFMDAITPT GTVITLLGLT PEGHRVAVHV YGTRQYFYMN KEEVDRHLQC RAPRDLCERM AAALRESPGA SF RGISADH FEAEVVERTD VYYYETRPAL FYRVYVRSGR VLSYLCDNFC PAIKKYEGGV DATTRFILDN PGFVTFGWYR LKP GRNNTL AQPRAPMAFG TSSDVEFNCT ADNLAIEGGM SDLPAYKLMC FDIECKAGGE DELAFPVAGH PEDLVIQISC LLYD LSTTA LEHVLLFSLG SCDLPESHLN ELAARGLPTP VVLEFDSEFE MLLAFMTLVK QYGPEFVTGY NIINFDWPFL LAKLT DIYK VPLDGYGRMN GRGVFRVWDI GQSHFQKRSK IKVNGMVNID MYGIITDKIK LSSYKLNAVA EAVLKDKKKD LSYRDI PAY YAAGPAQRGV IGEYCIQDSL LVGQLFFKFL PHLELSAVAR LAGINITRTI YDGQQIRVFT CLLRLADQKG FILPDTQ GR FRGAGGEAPK RPAAAREDEE RPEEEGEDED EREEGGGERE PEGARETAGR HVGYQGARVL DPTSGFHVNP VVVFDFAS L YPSIIQAHNL CFSTLSLRAD AVAHLEAGKD YLEIEVGGRR LFFVKAHVRE SLLSILLRDW LAMRKQIRSR IPQSSPEEA VLLDKQQAAI KVVCNSVYGF TGVQHGLLPC LHVAATVTTI GREMLLATRE YVHARWAAFE QLLADFPEAA DMRAPGPYSM RIIYGDTDS IFVLCRGLTA AGLTAVGDKM ASHISRALFL PPIKLECEKT FTKLLLIAKK KYIGVIYGGK MLIKGVDLVR K NNCAFINR TSRALVDLLF YDDTVSGAAA ALAERPAEEW LARPLPEGLQ AFGAVLVDAH RRITDPERDI QDFVLTAELS RH PRAYTNK RLAHLTVYYK LMARRAQVPS IKDRIPYVIV AQTREVEETV ARLAALRELD AAAPGDEPAP PAALPSPAKR PRE TPSPAD PPGGASKPRK LLVSELAEDP AYAIAHGVAL NTDYYFSHLL GAACVTFKAL FGNNAKITES LLKRFIPEVW HPPD DVAAR LRTAGFGAVG AGATAEETRR MLHRAFDTLA UniProtKB: DNA polymerase catalytic subunit |
-Macromolecule #2: DNA (46-MER)
| Macromolecule | Name: DNA (46-MER) / type: dna / ID: 2 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 13.96308 KDa |
| Sequence | String: (DG)(DC)(DC)(DA)(DC)(DT)(DA)(DC)(DG)(DA) (DC)(DA)(DC)(DC)(DT)(DT)(DG)(DA)(DT)(DC) (DG)(DC)(DC)(DT)(DC)(DG)(DC)(DA)(DG) (DC)(DC)(DG)(DT)(DC)(DC)(DA)(DA)(DC)(DC) (DA) (DA)(DC)(DT)(DC)(AS)(AS) |
-Macromolecule #3: DNA (67-MER)
| Macromolecule | Name: DNA (67-MER) / type: dna / ID: 3 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 20.902295 KDa |
| Sequence | String: (DA)(DT)(DT)(DT)(DG)(DC)(DT)(DG)(DA)(DC) (DC)(DT)(DT)(DT)(DG)(DT)(DT)(DC)(DT)(DG) (DG)(DG)(DT)(DG)(DA)(DG)(DT)(DT)(DG) (DG)(DT)(DT)(DG)(DG)(DA)(DC)(DG)(DG)(DC) (DT) (DG)(DC)(DG)(DA)(DG)(DG) ...String: (DA)(DT)(DT)(DT)(DG)(DC)(DT)(DG)(DA)(DC) (DC)(DT)(DT)(DT)(DG)(DT)(DT)(DC)(DT)(DG) (DG)(DG)(DT)(DG)(DA)(DG)(DT)(DT)(DG) (DG)(DT)(DT)(DG)(DG)(DA)(DC)(DG)(DG)(DC) (DT) (DG)(DC)(DG)(DA)(DG)(DG)(DC)(DG) (DA)(DT)(DC)(DA)(DA)(DG)(DG)(DT)(DG)(DT) (DC)(DG) (DT)(DA)(DG)(DT)(DG)(DG)(DC) |
-Macromolecule #4: CALCIUM ION
| Macromolecule | Name: CALCIUM ION / type: ligand / ID: 4 / Number of copies: 2 / Formula: CA |
|---|---|
| Molecular weight | Theoretical: 40.078 Da |
-Macromolecule #5: water
| Macromolecule | Name: water / type: ligand / ID: 5 / Number of copies: 7 / Formula: HOH |
|---|---|
| Molecular weight | Theoretical: 18.015 Da |
| Chemical component information |  ChemComp-HOH: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.4 mg/mL | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.8 Component:
Details: 20mM HEPES pH7.8, 150mM NaCl, 5mM CaCl2, 2mM DTT | |||||||||||||||
| Grid | Model: UltrAuFoil R1.2/1.3 / Material: GOLD / Mesh: 300 / Support film - Material: GOLD / Support film - topology: HOLEY | |||||||||||||||
| Vitrification | Cryogen name: ETHANE-PROPANE / Chamber humidity: 95 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 58.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 1.5 µm / Nominal defocus min: 0.3 µm / Nominal magnification: 165000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller




























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)