[English] 日本語
 Yorodumi
Yorodumi- EMDB-19792: Retron-Eco1 -1 turn mutant filament with ADP-ribosylated Effector... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Retron-Eco1 -1 turn mutant filament with ADP-ribosylated Effector (Consensus refinement) | |||||||||
 Map data Map data | Retron-Eco1 "-1 turn" mutant with ADPr. Consensus refinement. | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | retron / N-glycosidase / DNA-RNA-Protein complex / IMMUNE SYSTEM | |||||||||
| Function / homology |  Function and homology information Function and homology informationRNA-directed DNA polymerase / RNA-directed DNA polymerase activity / defense response to virus / RNA binding / metal ion binding Similarity search - Function | |||||||||
| Biological species |  | |||||||||
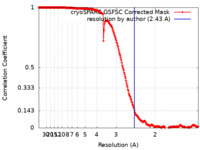
| Method | single particle reconstruction / cryo EM / Resolution: 2.43 Å | |||||||||
 Authors Authors | Carabias del Rey A / Montoya G / Pape T | |||||||||
| Funding support |  Denmark, 2 items Denmark, 2 items
| |||||||||
 Citation Citation |  Journal: Mol Cell / Year: 2024 Journal: Mol Cell / Year: 2024Title: Retron-Eco1 assembles NAD-hydrolyzing filaments that provide immunity against bacteriophages. Authors: Arturo Carabias / Sarah Camara-Wilpert / Mario Rodríguez Mestre / Blanca Lopéz-Méndez / Ivo A Hendriks / Ruiliang Zhao / Tillmann Pape / Anders Fuglsang / Sean Hoi-Ching Luk / Michael L ...Authors: Arturo Carabias / Sarah Camara-Wilpert / Mario Rodríguez Mestre / Blanca Lopéz-Méndez / Ivo A Hendriks / Ruiliang Zhao / Tillmann Pape / Anders Fuglsang / Sean Hoi-Ching Luk / Michael L Nielsen / Rafael Pinilla-Redondo / Guillermo Montoya /  Abstract: Retrons are toxin-antitoxin systems protecting bacteria against bacteriophages via abortive infection. The Retron-Eco1 antitoxin is formed by a reverse transcriptase (RT) and a non-coding RNA (ncRNA) ...Retrons are toxin-antitoxin systems protecting bacteria against bacteriophages via abortive infection. The Retron-Eco1 antitoxin is formed by a reverse transcriptase (RT) and a non-coding RNA (ncRNA)/multi-copy single-stranded DNA (msDNA) hybrid that neutralizes an uncharacterized toxic effector. Yet, the molecular mechanisms underlying phage defense remain unknown. Here, we show that the N-glycosidase effector, which belongs to the STIR superfamily, hydrolyzes NAD during infection. Cryoelectron microscopy (cryo-EM) analysis shows that the msDNA stabilizes a filament that cages the effector in a low-activity state in which ADPr, a NAD hydrolysis product, is covalently linked to the catalytic E106 residue. Mutations shortening the msDNA induce filament disassembly and the effector's toxicity, underscoring the msDNA role in immunity. Furthermore, we discovered a phage-encoded Retron-Eco1 inhibitor (U56) that binds ADPr, highlighting the intricate interplay between retron systems and phage evolution. Our work outlines the structural basis of Retron-Eco1 defense, uncovering ADPr's pivotal role in immunity. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_19792.map.gz emd_19792.map.gz | 1 GB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-19792-v30.xml emd-19792-v30.xml emd-19792.xml emd-19792.xml | 24 KB 24 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_19792_fsc.xml emd_19792_fsc.xml | 22.6 KB | Display |  FSC data file FSC data file |
| Images |  emd_19792.png emd_19792.png | 82.9 KB | ||
| Filedesc metadata |  emd-19792.cif.gz emd-19792.cif.gz | 6.5 KB | ||
| Others |  emd_19792_half_map_1.map.gz emd_19792_half_map_1.map.gz emd_19792_half_map_2.map.gz emd_19792_half_map_2.map.gz | 1.1 GB 1.1 GB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-19792 http://ftp.pdbj.org/pub/emdb/structures/EMD-19792 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-19792 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-19792 | HTTPS FTP |
-Related structure data
| Related structure data |  8qbkC  8qblC  8qbmC C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_19792.map.gz / Format: CCP4 / Size: 1.2 GB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_19792.map.gz / Format: CCP4 / Size: 1.2 GB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Retron-Eco1 "-1 turn" mutant with ADPr. Consensus refinement. | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.725 Å | ||||||||||||||||||||||||||||||||||||
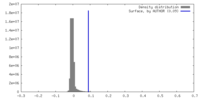
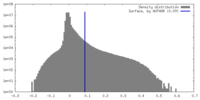
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: Retron-Eco1 "-1 turn" mutant with ADPr. Consensus refinement....
| File | emd_19792_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Retron-Eco1 "-1 turn" mutant with ADPr. Consensus refinement. Half map B. | ||||||||||||
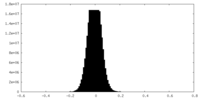
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Retron-Eco1 "-1 turn" mutant with ADPr. Consensus refinement....
| File | emd_19792_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Retron-Eco1 "-1 turn" mutant with ADPr. Consensus refinement. Half map A. | ||||||||||||
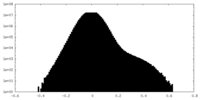
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Filamentous assembly of Retron-Eco1 "-1 turn" mutant in complex w...
| Entire | Name: Filamentous assembly of Retron-Eco1 "-1 turn" mutant in complex with ADPr, containing RT-msr-msDNA-Effector complex (Consensus refinement) |
|---|---|
| Components |
|
-Supramolecule #1: Filamentous assembly of Retron-Eco1 "-1 turn" mutant in complex w...
| Supramolecule | Name: Filamentous assembly of Retron-Eco1 "-1 turn" mutant in complex with ADPr, containing RT-msr-msDNA-Effector complex (Consensus refinement) type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#5 / Details: Filament formed by addition of NAD+ |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 21 kDa/nm |
-Macromolecule #1: Retron-Eco1 Reverse Transcriptase
| Macromolecule | Name: Retron-Eco1 Reverse Transcriptase / type: protein_or_peptide / ID: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Recombinant expression | Organism:  |
| Sequence | String: MKSAEYLNTF RLRNLGLPVM NNLHDMSKAT RISVETLRLL IYTADFRYRI YTVEKKGPEK RMRTIYQPSR ELKALQGWVL RNILDKLSSS PFSIGFEKHQ SILNNATPHI GANFILNIDL EDFFPSLTAN KVFGVFHSLG YNRLISSVLT KICCYKNLLP QGAPSSPKLA ...String: MKSAEYLNTF RLRNLGLPVM NNLHDMSKAT RISVETLRLL IYTADFRYRI YTVEKKGPEK RMRTIYQPSR ELKALQGWVL RNILDKLSSS PFSIGFEKHQ SILNNATPHI GANFILNIDL EDFFPSLTAN KVFGVFHSLG YNRLISSVLT KICCYKNLLP QGAPSSPKLA NLICSKLDYR IQGYAGSRGL IYTRYADDLT LSAQSMKKVV KARDFLFSII PSEGLVINSK KTCISGPRSQ RKVTGLVISQ EKVGIGREKY KEIRAKIHHI FCGKSSEIEH VRGWLSFILS VDSKSHRRLI TYISKLEKKY GKNPLNKAKT GSEFELENLY FQGELRRQAS ALEHHHHHH UniProtKB: Retron Ec86 reverse transcriptase |
-Macromolecule #4: Retron-Eco1-Effector
| Macromolecule | Name: Retron-Eco1-Effector / type: protein_or_peptide / ID: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Recombinant expression | Organism:  |
| Sequence | String: MNKKFTDEQQ QQLIGHLTKK GFYRGANIKI TIFLCGGDVA NHQSWRHQLS QFLAKFSDVD IFYPEDLFDD LLAGQGQHSL LSLENILAEA VDVIILFPES PGSFTELGAF SNNENLRRKL ICIQDAKFKS KRSFINYGPV RLLRKFNSKS VLRCSSNELK EMCDSSIDVA ...String: MNKKFTDEQQ QQLIGHLTKK GFYRGANIKI TIFLCGGDVA NHQSWRHQLS QFLAKFSDVD IFYPEDLFDD LLAGQGQHSL LSLENILAEA VDVIILFPES PGSFTELGAF SNNENLRRKL ICIQDAKFKS KRSFINYGPV RLLRKFNSKS VLRCSSNELK EMCDSSIDVA RKLRLYKKLM ASIKKVRKEN KVSKDIGNIL YAERFLLPCI YLLDSVNYRT LCELAFKAIK QDDVLSKIIV RSVVSRLINE RKILQMTDGY QVTALGASYV RSVFDRKTLD RLRLEIMNFE NRRKSTFNYD KIPYAHP UniProtKB: Retron Ec86 putative ribosyltransferase/DNA-binding protein |
-Macromolecule #2: Retron-Eco1-msr
| Macromolecule | Name: Retron-Eco1-msr / type: rna / ID: 2 |
|---|---|
| Source (natural) | Organism:  |
| Sequence | String: AUGCGCACCC UUAGCGAGAG GUUUAUCAUU AAGGUCAACC UCUGGAUGUU GUUUCGGCAU CCUGCAUUGA AUCUGAGUUA CU |
-Macromolecule #3: Retron-Eco1-A2
| Macromolecule | Name: Retron-Eco1-A2 / type: rna / ID: 3 |
|---|---|
| Source (natural) | Organism:  |
| Sequence | String: CGUAAGGGUG CGCA |
-Macromolecule #5: Retron-Eco1-msDNA "-1 turn"
| Macromolecule | Name: Retron-Eco1-msDNA "-1 turn" / type: dna / ID: 5 / Classification: DNA |
|---|---|
| Source (natural) | Organism:  |
| Sequence | String: gtcagaaaaa acgggtttcc tggttggctc AGGCgttcca acaaggaaaa cagacagtaa ctcag |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | filament |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 Component:
Details: 20 mM HEPES (pH 7.5),200 mM KCl, 5 mM MgCl2, 1mM TCEP | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 60 sec. / Pretreatment - Atmosphere: AIR / Pretreatment - Pressure: 0.03 kPa / Details: 10 mAmps | ||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV / Details: Blotting time 3 seconds. | ||||||||||
| Details | The complex was prepared at a concentration corresponding to A260nm = ~12 |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Temperature | Min: 100.0 K / Max: 100.0 K |
| Specialist optics | Energy filter - Name: TFS Selectris X / Energy filter - Slit width: 5 eV Details: The slit was recentered every 6 hours during data collection |
| Image recording | Film or detector model: TFS FALCON 4i (4k x 4k) / Detector mode: COUNTING / Digitization - Dimensions - Width: 4096 pixel / Digitization - Dimensions - Height: 4096 pixel / Number grids imaged: 1 / Number real images: 26559 / Average exposure time: 2.6 sec. / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.4 µm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model | PDB ID: Chain - Source name: PDB / Chain - Initial model type: experimental model |
|---|---|
| Details | in ChimeraX |
| Refinement | Space: REAL / Protocol: RIGID BODY FIT |
 Movie
Movie Controller
Controller








 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)