[English] 日本語
 Yorodumi
Yorodumi- EMDB-19255: Human Replication protein A (RPA; trimeric core) - ssDNA complex -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Human Replication protein A (RPA; trimeric core) - ssDNA complex | |||||||||||||||||||||||||||
 Map data Map data | ||||||||||||||||||||||||||||
 Sample Sample |
| |||||||||||||||||||||||||||
 Keywords Keywords | ssDNA binding protein / DNA damage repair / Single-strand annealing / DNA BINDING PROTEIN | |||||||||||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationprotein localization to chromosome / DNA replication factor A complex / single-stranded telomeric DNA binding / lateral element / Removal of the Flap Intermediate / regulation of DNA damage checkpoint / protein localization to site of double-strand break / G-rich strand telomeric DNA binding / Mismatch repair (MMR) directed by MSH2:MSH3 (MutSbeta) / Mismatch repair (MMR) directed by MSH2:MSH6 (MutSalpha) ...protein localization to chromosome / DNA replication factor A complex / single-stranded telomeric DNA binding / lateral element / Removal of the Flap Intermediate / regulation of DNA damage checkpoint / protein localization to site of double-strand break / G-rich strand telomeric DNA binding / Mismatch repair (MMR) directed by MSH2:MSH3 (MutSbeta) / Mismatch repair (MMR) directed by MSH2:MSH6 (MutSalpha) / chromatin-protein adaptor activity / Removal of the Flap Intermediate from the C-strand / HDR through Single Strand Annealing (SSA) / regulation of double-strand break repair via homologous recombination / Impaired BRCA2 binding to RAD51 / telomeric DNA binding / hemopoiesis / Presynaptic phase of homologous DNA pairing and strand exchange / PCNA-Dependent Long Patch Base Excision Repair / Activation of the pre-replicative complex / Regulation of HSF1-mediated heat shock response / HSF1 activation / Activation of ATR in response to replication stress / site of DNA damage / telomere maintenance via telomerase / mismatch repair / SUMOylation of DNA damage response and repair proteins / mitotic G1 DNA damage checkpoint signaling / homeostasis of number of cells within a tissue / regulation of mitotic cell cycle / telomere maintenance / Translesion synthesis by REV1 / Translesion synthesis by POLK / Translesion synthesis by POLI / Gap-filling DNA repair synthesis and ligation in GG-NER / male germ cell nucleus / meiotic cell cycle / nucleotide-excision repair / Fanconi Anemia Pathway / Termination of translesion DNA synthesis / Translesion Synthesis by POLH / Recognition of DNA damage by PCNA-containing replication complex / double-strand break repair via homologous recombination / PML body / G2/M DNA damage checkpoint / base-excision repair / DNA-templated DNA replication / HDR through Homologous Recombination (HRR) / Meiotic recombination / Dual Incision in GG-NER / Formation of Incision Complex in GG-NER / Dual incision in TC-NER / Gap-filling DNA repair synthesis and ligation in TC-NER / regulation of cell population proliferation / single-stranded DNA binding / site of double-strand break / Processing of DNA double-strand break ends / protein phosphatase binding / DNA recombination / Regulation of TP53 Activity through Phosphorylation / in utero embryonic development / damaged DNA binding / DNA replication / chromosome, telomeric region / nuclear body / DNA repair / positive regulation of cell population proliferation / DNA damage response / ubiquitin protein ligase binding / chromatin binding / chromatin / enzyme binding / zinc ion binding / nucleoplasm / nucleus Similarity search - Function | |||||||||||||||||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||||||||||||||||||||
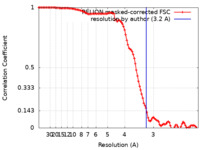
| Method | single particle reconstruction / cryo EM / Resolution: 3.2 Å | |||||||||||||||||||||||||||
 Authors Authors | Liang CC / West SC | |||||||||||||||||||||||||||
| Funding support |  United Kingdom, European Union, United Kingdom, European Union,  Switzerland, 8 items Switzerland, 8 items
| |||||||||||||||||||||||||||
 Citation Citation |  Journal: Nature / Year: 2024 Journal: Nature / Year: 2024Title: Mechanism of single-stranded DNA annealing by RAD52-RPA complex. Authors: Chih-Chao Liang / Luke A Greenhough / Laura Masino / Sarah Maslen / Ilirjana Bajrami / Marcel Tuppi / Mark Skehel / Ian A Taylor / Stephen C West /  Abstract: RAD52 is important for the repair of DNA double-stranded breaks, mitotic DNA synthesis and alternative telomere length maintenance. Central to these functions, RAD52 promotes the annealing of ...RAD52 is important for the repair of DNA double-stranded breaks, mitotic DNA synthesis and alternative telomere length maintenance. Central to these functions, RAD52 promotes the annealing of complementary single-stranded DNA (ssDNA) and provides an alternative to BRCA2/RAD51-dependent homologous recombination repair. Inactivation of RAD52 in homologous-recombination-deficient BRCA1- or BRCA2-defective cells is synthetically lethal, and aberrant expression of RAD52 is associated with poor cancer prognosis. As a consequence, RAD52 is an attractive therapeutic target against homologous-recombination-deficient breast, ovarian and prostate cancers. Here we describe the structure of RAD52 and define the mechanism of annealing. As reported previously, RAD52 forms undecameric (11-subunit) ring structures, but these rings do not represent the active form of the enzyme. Instead, cryo-electron microscopy and biochemical analyses revealed that ssDNA annealing is driven by RAD52 open rings in association with replication protein-A (RPA). Atomic models of the RAD52-ssDNA complex show that ssDNA sits in a positively charged channel around the ring. Annealing is driven by the RAD52 N-terminal domains, whereas the C-terminal regions modulate the open-ring conformation and RPA interaction. RPA associates with RAD52 at the site of ring opening with critical interactions occurring between the RPA-interacting domain of RAD52 and the winged helix domain of RPA2. Our studies provide structural snapshots throughout the annealing process and define the molecular mechanism of ssDNA annealing by the RAD52-RPA complex. | |||||||||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_19255.map.gz emd_19255.map.gz | 6.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-19255-v30.xml emd-19255-v30.xml emd-19255.xml emd-19255.xml | 21.4 KB 21.4 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_19255_fsc.xml emd_19255_fsc.xml | 10.6 KB | Display |  FSC data file FSC data file |
| Images |  emd_19255.png emd_19255.png | 55.2 KB | ||
| Filedesc metadata |  emd-19255.cif.gz emd-19255.cif.gz | 6.8 KB | ||
| Others |  emd_19255_half_map_1.map.gz emd_19255_half_map_1.map.gz emd_19255_half_map_2.map.gz emd_19255_half_map_2.map.gz | 80.8 MB 80.8 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-19255 http://ftp.pdbj.org/pub/emdb/structures/EMD-19255 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-19255 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-19255 | HTTPS FTP |
-Related structure data
| Related structure data |  8rk2MC  8rilC  8rj3C  8rjwC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_19255.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_19255.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.08 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_19255_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_19255_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Open ring conformation of human RAD52 in complex with ssDNA
| Entire | Name: Open ring conformation of human RAD52 in complex with ssDNA |
|---|---|
| Components |
|
-Supramolecule #1: Open ring conformation of human RAD52 in complex with ssDNA
| Supramolecule | Name: Open ring conformation of human RAD52 in complex with ssDNA type: complex / ID: 1 / Parent: 0 / Macromolecule list: all Details: Recombinant RAD52 purified from E. coli, and the open ring conformation was separated by cation exchange. The RAD52-ssDNA complex was reconstituted in vitro. |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Replication protein A 70 kDa DNA-binding subunit, N-terminally pr...
| Macromolecule | Name: Replication protein A 70 kDa DNA-binding subunit, N-terminally processed type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 68.212977 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MVGQLSEGAI AAIMQKGDTN IKPILQVINI RPITTGNSPP RYRLLMSDGL NTLSSFMLAT QLNPLVEEEQ LSSNCVCQIH RFIVNTLKD GRRVVILMEL EVLKSAEAVG VKIGNPVPYN EGLGQPQVAP PAPAASPAAS SRPQPQNGSS GMGSTVSKAY G ASKTFGKA ...String: MVGQLSEGAI AAIMQKGDTN IKPILQVINI RPITTGNSPP RYRLLMSDGL NTLSSFMLAT QLNPLVEEEQ LSSNCVCQIH RFIVNTLKD GRRVVILMEL EVLKSAEAVG VKIGNPVPYN EGLGQPQVAP PAPAASPAAS SRPQPQNGSS GMGSTVSKAY G ASKTFGKA AGPSLSHTSG GTQSKVVPIA SLTPYQSKWT ICARVTNKSQ IRTWSNSRGE GKLFSLELVD ESGEIRATAF NE QVDKFFP LIEVNKVYYF SKGTLKIANK QFTAVKNDYE MTFNNETSVM PCEDDHHLPT VQFDFTGIDD LENKSKDSLV DII GICKSY EDATKITVRS NNREVAKRNI YLMDTSGKVV TATLWGEDAD KFDGSRQPVL AIKGARVSDF GGRSLSVLSS STII ANPDI PEAYKLRGWF DAEGQALDGV SISDLKSGGV GGSNTNWKTL YEVKSENLGQ GDKPDYFSSV ATVVYLRKEN CMYQA CPTQ DCNKKVIDQQ NGLYRCEKCD TEFPNFKYRM ILSVNIADFQ ENQWVTCFQE SAEAILGQNA AYLGELKDKN EQAFEE VFQ NANFRSFIFR VRVKVETYND ESRIKATVMD VKPVDYREYG RRLVMSIRRS ALM UniProtKB: Replication protein A 70 kDa DNA-binding subunit |
-Macromolecule #2: Replication protein A 32 kDa subunit
| Macromolecule | Name: Replication protein A 32 kDa subunit / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 29.276795 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MWNSGFESYG SSSYGGAGGY TQSPGGFGSP APSQAEKKSR ARAQHIVPCT ISQLLSATLV DEVFRIGNVE ISQVTIVGII RHAEKAPTN IVYKIDDMTA APMDVRQWVD TDDTSSENTV VPPETYVKVA GHLRSFQNKK SLVAFKIMPL EDMNEFTTHI L EVINAHMV ...String: MWNSGFESYG SSSYGGAGGY TQSPGGFGSP APSQAEKKSR ARAQHIVPCT ISQLLSATLV DEVFRIGNVE ISQVTIVGII RHAEKAPTN IVYKIDDMTA APMDVRQWVD TDDTSSENTV VPPETYVKVA GHLRSFQNKK SLVAFKIMPL EDMNEFTTHI L EVINAHMV LSKANSQPSA GRAPISNPGM SEAGNFGGNS FMPANGLTVA QNQVLNLIKA CPRPEGLNFQ DLKNQLKHMS VS SIKQAVD FLSNEGHIYS TVDDDHFKST DAE UniProtKB: Replication protein A 32 kDa subunit |
-Macromolecule #3: Replication protein A 14 kDa subunit
| Macromolecule | Name: Replication protein A 14 kDa subunit / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 13.583714 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MVDMMDLPRS RINAGMLAQF IDKPVCFVGR LEKIHPTGKM FILSDGEGKN GTIELMEPLD EEISGIVEVV GRVTAKATIL CTSYVQFKE DSHPFDLGLY NEAVKIIHDF PQFYPLGIVQ HD UniProtKB: Replication protein A 14 kDa subunit |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.15 mg/mL | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
| ||||||||||||||||||
| Grid | Model: UltrAuFoil R2/2 / Material: GOLD / Mesh: 200 | ||||||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 95 % / Chamber temperature: 277.15 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 QUANTUM (4k x 4k) / Detector mode: COUNTING / Average electron dose: 46.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 3.5 µm / Nominal defocus min: 1.5 µm / Nominal magnification: 130000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model | Chain - Source name: AlphaFold / Chain - Initial model type: in silico model |
|---|---|
| Refinement | Space: REAL / Protocol: RIGID BODY FIT |
| Output model |  PDB-8rk2: |
 Movie
Movie Controller
Controller



















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)