+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | consensus map of the ASFV RNA polymerase in open conformation | ||||||||||||
 Map data Map data | 3D refinement map (not sharpened) | ||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | RNA polymerase / ASFV / transcription / eukaryotic virus | ||||||||||||
| Biological species |  African swine fever virus BA71V African swine fever virus BA71V | ||||||||||||
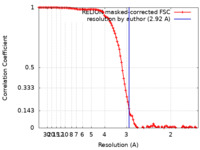
| Method | single particle reconstruction / cryo EM / Resolution: 2.92 Å | ||||||||||||
 Authors Authors | Pilotto S / Sykora M / Cackett G / Werner F | ||||||||||||
| Funding support |  United Kingdom, 3 items United Kingdom, 3 items
| ||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: Structure of the recombinant RNA polymerase from African Swine Fever Virus. Authors: Simona Pilotto / Michal Sýkora / Gwenny Cackett / Christopher Dulson / Finn Werner /  Abstract: African Swine Fever Virus is a Nucleo-Cytoplasmic Large DNA Virus that causes an incurable haemorrhagic fever in pigs with a high impact on global food security. ASFV replicates in the cytoplasm of ...African Swine Fever Virus is a Nucleo-Cytoplasmic Large DNA Virus that causes an incurable haemorrhagic fever in pigs with a high impact on global food security. ASFV replicates in the cytoplasm of the infected cell and encodes its own transcription machinery that is independent of cellular factors, however, not much is known about how this system works at a molecular level. Here, we present methods to produce recombinant ASFV RNA polymerase, functional assays to screen for inhibitors, and high-resolution cryo-electron microscopy structures of the ASFV RNAP in different conformational states. The ASFV RNAP bears a striking resemblance to RNAPII with bona fide homologues of nine of its twelve subunits. Key differences include the fusion of the ASFV assembly platform subunits RPB3 and RPB11, and an unusual C-terminal domain of the stalk subunit vRPB7 that is related to the eukaryotic mRNA cap 2´-O-methyltransferase 1. Despite the high degree of structural conservation with cellular RNA polymerases, the ASFV RNAP is resistant to the inhibitors rifampicin and alpha-amanitin. The cryo-EM structures and fully recombinant RNAP system together provide an important tool for the design, development, and screening of antiviral drugs in a low biosafety containment environment. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_18163.map.gz emd_18163.map.gz | 80.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-18163-v30.xml emd-18163-v30.xml emd-18163.xml emd-18163.xml | 18.8 KB 18.8 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_18163_fsc.xml emd_18163_fsc.xml | 10.7 KB | Display |  FSC data file FSC data file |
| Images |  emd_18163.png emd_18163.png | 105.1 KB | ||
| Filedesc metadata |  emd-18163.cif.gz emd-18163.cif.gz | 5.1 KB | ||
| Others |  emd_18163_half_map_1.map.gz emd_18163_half_map_1.map.gz emd_18163_half_map_2.map.gz emd_18163_half_map_2.map.gz | 81 MB 81 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-18163 http://ftp.pdbj.org/pub/emdb/structures/EMD-18163 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-18163 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-18163 | HTTPS FTP |
-Validation report
| Summary document |  emd_18163_validation.pdf.gz emd_18163_validation.pdf.gz | 969.3 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_18163_full_validation.pdf.gz emd_18163_full_validation.pdf.gz | 968.9 KB | Display | |
| Data in XML |  emd_18163_validation.xml.gz emd_18163_validation.xml.gz | 17.6 KB | Display | |
| Data in CIF |  emd_18163_validation.cif.gz emd_18163_validation.cif.gz | 22.8 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-18163 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-18163 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-18163 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-18163 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_18163.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_18163.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | 3D refinement map (not sharpened) | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.828 Å | ||||||||||||||||||||||||||||||||||||
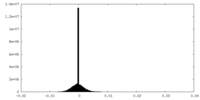
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: half 2 map
| File | emd_18163_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half 2 map | ||||||||||||
| Projections & Slices |
| ||||||||||||
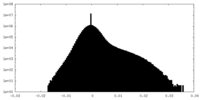
| Density Histograms |
-Half map: half 1 map
| File | emd_18163_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half 1 map | ||||||||||||
| Projections & Slices |
| ||||||||||||
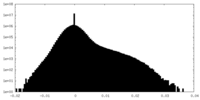
| Density Histograms |
- Sample components
Sample components
-Entire : apo-form of the 8-subunit RNA polymerase from African Swine Fever...
| Entire | Name: apo-form of the 8-subunit RNA polymerase from African Swine Fever Virus |
|---|---|
| Components |
|
-Supramolecule #1: apo-form of the 8-subunit RNA polymerase from African Swine Fever...
| Supramolecule | Name: apo-form of the 8-subunit RNA polymerase from African Swine Fever Virus type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#8 |
|---|---|
| Source (natural) | Organism:  African swine fever virus BA71V African swine fever virus BA71V |
| Molecular weight | Theoretical: 450 KDa |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.05 mg/mL | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
| ||||||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 95 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV | ||||||||||||||||||
| Details | This sample was monodisparse |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number real images: 14638 / Average exposure time: 2.6 sec. / Average electron dose: 48.152 e/Å2 Details: Images were collected in movie-mode for a total of 50 frames per image. The data collection was carried out in super-resolution mode and binned 2 on-the-fly. |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.7 µm / Nominal defocus min: 1.5 µm / Nominal magnification: 105000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model | PDB ID: Chain - Source name: Other / Chain - Initial model type: experimental model Details: The complete biological assembly for the PDB entry 8Q3B was used for the docking in the new map |
|---|---|
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT / Overall B value: 84.5995 / Target criteria: cross-correlation coefficient |
 Movie
Movie Controller
Controller









 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)