[English] 日本語
 Yorodumi
Yorodumi- EMDB-1802: Conformational flexibility of RNA polymerase III during transcrip... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-1802 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Conformational flexibility of RNA polymerase III during transcriptional elongation | |||||||||
 Map data Map data | The map represents the RNA-polymerase III. | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | transcription / RNA polymerase III / elongation complex / transcription initiation / transcription elongation / transcription termination | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 11.5 Å | |||||||||
 Authors Authors | Fernandez-Tornero C / Bottcher B / Rashid UJ / Steuerwald U / Florchinger B / Devos DP / Lindner D / Muller CW | |||||||||
 Citation Citation |  Journal: EMBO J / Year: 2010 Journal: EMBO J / Year: 2010Title: Conformational flexibility of RNA polymerase III during transcriptional elongation. Authors: Carlos Fernández-Tornero / Bettina Böttcher / Umar Jan Rashid / Ulrich Steuerwald / Beate Flörchinger / Damien P Devos / Doris Lindner / Christoph W Müller /  Abstract: RNA polymerase (Pol) III is responsible for the transcription of genes encoding small RNAs, including tRNA, 5S rRNA and U6 RNA. Here, we report the electron cryomicroscopy structures of yeast Pol III ...RNA polymerase (Pol) III is responsible for the transcription of genes encoding small RNAs, including tRNA, 5S rRNA and U6 RNA. Here, we report the electron cryomicroscopy structures of yeast Pol III at 9.9 Å resolution and its elongation complex at 16.5 Å resolution. Particle sub-classification reveals prominent EM densities for the two Pol III-specific subcomplexes, C31/C82/C34 and C37/C53, that can be interpreted using homology models. While the winged-helix-containing C31/C82/C34 subcomplex initiates transcription from one side of the DNA-binding cleft, the C37/C53 subcomplex accesses the transcription bubble from the opposite side of this cleft. The transcribing Pol III enzyme structure not only shows the complete incoming DNA duplex, but also reveals the exit path of newly synthesized RNA. During transcriptional elongation, the Pol III-specific subcomplexes tightly enclose the incoming DNA duplex, which likely increases processivity and provides structural insights into the conformational switch between Pol III-mediated initiation and elongation. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_1802.map.gz emd_1802.map.gz | 20.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-1802-v30.xml emd-1802-v30.xml emd-1802.xml emd-1802.xml | 10 KB 10 KB | Display Display |  EMDB header EMDB header |
| Images |  1802.png 1802.png | 139 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-1802 http://ftp.pdbj.org/pub/emdb/structures/EMD-1802 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-1802 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-1802 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_1802.map.gz / Format: CCP4 / Size: 21.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_1802.map.gz / Format: CCP4 / Size: 21.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | The map represents the RNA-polymerase III. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.4 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
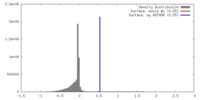
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : RNA Polymerase III
| Entire | Name: RNA Polymerase III |
|---|---|
| Components |
|
-Supramolecule #1000: RNA Polymerase III
| Supramolecule | Name: RNA Polymerase III / type: sample / ID: 1000 / Details: Sample was monodisperse / Oligomeric state: One complex of 17 different polypeptides / Number unique components: 1 |
|---|---|
| Molecular weight | Experimental: 700 KDa / Theoretical: 700 KDa / Method: Native mass spectrometry |
-Macromolecule #1: RNA polymerase III
| Macromolecule | Name: RNA polymerase III / type: protein_or_peptide / ID: 1 / Name.synonym: Pol III / Number of copies: 1 / Oligomeric state: Complex of 17 subunits / Recombinant expression: Yes |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Experimental: 700 KDa / Theoretical: 700 KDa |
| Recombinant expression | Organism:  |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.5 mg/mL |
|---|---|
| Buffer | pH: 7.4 Details: 10 mM Tris-HCl pH 7.4 150 mM Ammonium sulphate 10 mM DTT |
| Grid | Details: 400 mesh copper rhodium grids coated with perforated carbon film |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Instrument: HOMEMADE PLUNGER Details: Vitrification instrument: Controlled environment, self-built Method: Blot for 15 s in controlled environment chamber before plunging |
- Electron microscopy
Electron microscopy
| Microscope | FEI/PHILIPS CM200FEG |
|---|---|
| Temperature | Average: 95 K |
| Alignment procedure | Legacy - Astigmatism: Astigmatism was corrected at 200,000 times magnification |
| Image recording | Category: FILM / Film or detector model: KODAK SO-163 FILM / Digitization - Scanner: ZEISS SCAI / Digitization - Sampling interval: 7 µm / Number real images: 593 / Bits/pixel: 8 |
| Tilt angle min | 0 |
| Tilt angle max | 0 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2 mm / Nominal defocus max: 5.0 µm / Nominal defocus min: 1.6 µm / Nominal magnification: 50000 |
| Sample stage | Specimen holder: Eucentric / Specimen holder model: GATAN LIQUID NITROGEN |
- Image processing
Image processing
| Details | ca. 100000 particles were sorted into three groups according to compositional differences. The reconstruction shows the most complete species and represents ca. 48% of the particle population. |
|---|---|
| CTF correction | Details: Each particle |
| Final reconstruction | Applied symmetry - Point group: C1 (asymmetric) / Algorithm: OTHER / Resolution.type: BY AUTHOR / Resolution: 11.5 Å / Resolution method: FSC 0.5 CUT-OFF / Software - Name: SPIDER Details: Maps were calculated from defocus groups,and added. final combined map was amplitude corrected Number images used: 40200 |
 Movie
Movie Controller
Controller











 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)