+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
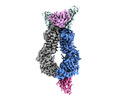
| Title | Structure of 5D3-Fab and nanobody(Nb17)-bound ABCG2 | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationbiotin transmembrane transporter activity / biotin transport / riboflavin transport / riboflavin transmembrane transporter activity / sphingolipid transporter activity / renal urate salt excretion / Abacavir transmembrane transport / urate metabolic process / sphingolipid biosynthetic process / Sphingolipid de novo biosynthesis ...biotin transmembrane transporter activity / biotin transport / riboflavin transport / riboflavin transmembrane transporter activity / sphingolipid transporter activity / renal urate salt excretion / Abacavir transmembrane transport / urate metabolic process / sphingolipid biosynthetic process / Sphingolipid de novo biosynthesis / urate transmembrane transporter activity / external side of apical plasma membrane / xenobiotic transport across blood-brain barrier / organic anion transport / : / transepithelial transport / Ciprofloxacin ADME / Paracetamol ADME / export across plasma membrane / NFE2L2 regulating MDR associated enzymes / Differentiation of Keratinocytes in Interfollicular Epidermis in Mammalian Skin / ABC-type xenobiotic transporter / Heme biosynthesis / cellular detoxification / ABC-type xenobiotic transporter activity / Heme degradation / efflux transmembrane transporter activity / ATPase-coupled transmembrane transporter activity / xenobiotic transmembrane transporter activity / transport across blood-brain barrier / brush border membrane / Iron uptake and transport / transmembrane transport / mitochondrial membrane / apical plasma membrane / membrane raft / protein homodimerization activity / ATP hydrolysis activity / nucleoplasm / ATP binding / identical protein binding / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /   | |||||||||
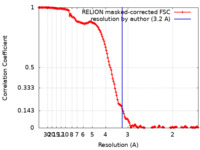
| Method | single particle reconstruction / cryo EM / Resolution: 3.2 Å | |||||||||
 Authors Authors | Irobalieva RN / Manolaridis I / Jackson SM / Ni D / Pardon E / Stahlberg H / Steyaert J / Locher KP | |||||||||
| Funding support |  Switzerland, 1 items Switzerland, 1 items
| |||||||||
 Citation Citation |  Journal: J Mol Biol / Year: 2023 Journal: J Mol Biol / Year: 2023Title: Structural Basis of the Allosteric Inhibition of Human ABCG2 by Nanobodies. Authors: Rossitza N Irobalieva / Ioannis Manolaridis / Scott M Jackson / Dongchun Ni / Els Pardon / Henning Stahlberg / Jan Steyaert / Kaspar P Locher /   Abstract: ABCG2 is an ATP-binding cassette transporter that exports a wide range of xenobiotic compounds and has been recognized as a contributing factor for multidrug resistance in cancer cells. Substrate and ...ABCG2 is an ATP-binding cassette transporter that exports a wide range of xenobiotic compounds and has been recognized as a contributing factor for multidrug resistance in cancer cells. Substrate and inhibitor interactions with ABCG2 have been extensively studied and small molecule inhibitors have been developed that prevent the export of anticancer drugs from tumor cells. Here, we explore the potential for inhibitors that target sites other than the substrate binding pocket of ABCG2. We developed novel nanobodies against ABCG2 and used functional analyses to select three inhibitory nanobodies (Nb8, Nb17 and Nb96) for structural studies by single particle cryo-electron microscopy. Our results showed that these nanobodies allosterically bind to different regions of the nucleotide binding domains. Two copies of Nb8 bind to the apex of the NBDs preventing them from fully closing. Nb17 binds near the two-fold axis of the transporter and interacts with both NBDs. Nb96 binds to the side of the NBD and immobilizes a region connected to key motifs involved in ATP binding and hydrolysis. All three nanobodies prevent the transporter from undergoing conformational changes required for substrate transport. These findings advance our understanding of the molecular basis of modulation of ABCG2 by external binders, which may contribute to the development of a new generation of inhibitors. Furthermore, this is the first example of modulation of human multidrug resistance transporters by nanobodies. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_17543.map.gz emd_17543.map.gz | 15.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-17543-v30.xml emd-17543-v30.xml emd-17543.xml emd-17543.xml | 20.5 KB 20.5 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_17543_fsc.xml emd_17543_fsc.xml | 14.2 KB | Display |  FSC data file FSC data file |
| Images |  emd_17543.png emd_17543.png | 70.1 KB | ||
| Filedesc metadata |  emd-17543.cif.gz emd-17543.cif.gz | 6.8 KB | ||
| Others |  emd_17543_half_map_1.map.gz emd_17543_half_map_1.map.gz emd_17543_half_map_2.map.gz emd_17543_half_map_2.map.gz | 194.9 MB 195 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-17543 http://ftp.pdbj.org/pub/emdb/structures/EMD-17543 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-17543 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-17543 | HTTPS FTP |
-Related structure data
| Related structure data |  8p8aMC  8p7wC  8p8jC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_17543.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_17543.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.84 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_17543_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_17543_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Structure of nanobody (Nb8)-bound ABCG2
| Entire | Name: Structure of nanobody (Nb8)-bound ABCG2 |
|---|---|
| Components |
|
-Supramolecule #1: Structure of nanobody (Nb8)-bound ABCG2
| Supramolecule | Name: Structure of nanobody (Nb8)-bound ABCG2 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: 5D3(Fab) light chain variable domain
| Macromolecule | Name: 5D3(Fab) light chain variable domain / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 23.594016 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: DIVLTQSPSS FSVSLGDRVT ISCKASGYIL NRLAWYQQKP GNAPRLLISG ATSLETGFPS RFSGTGSGKD YTLSISSLQT EDVGTYYCQ QYWSTPWTFG GGTKLEIRRA DAAPTVSIFP PSSEQLTSGG ASVVCFLNNF YPKDINVKWK IDGSERQNGV L NSWTDQDS ...String: DIVLTQSPSS FSVSLGDRVT ISCKASGYIL NRLAWYQQKP GNAPRLLISG ATSLETGFPS RFSGTGSGKD YTLSISSLQT EDVGTYYCQ QYWSTPWTFG GGTKLEIRRA DAAPTVSIFP PSSEQLTSGG ASVVCFLNNF YPKDINVKWK IDGSERQNGV L NSWTDQDS KDSTYSMSST LTLTKDEYER HNSYTCEATH KTSTSPIVKS FNRNEC |
-Macromolecule #2: 5D3(Fab) heavy chain variable domain
| Macromolecule | Name: 5D3(Fab) heavy chain variable domain / type: protein_or_peptide / ID: 2 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 17.508617 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: QVQLQESGPG LVKPSQSLSL TCTVTGFSIT SDYAWNWIRQ FPGKKLEWMG YINFDGGTTY NPSLRGRISI TRDTSKNQFF LQLRSVTPE DTATYYCATF YGAKGTLDYW GQGTSVTVSS AKTTPPSVYP LAPVCGDTSG SSVTLGCLVK GYFPEPVTLT W |
-Macromolecule #3: Nanobody
| Macromolecule | Name: Nanobody / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 13.384795 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: QVQLQESGGG LVQAGGSLRL SCTASGRTFG SYAMGWFRQA PGKDREFVAA ISTTGRSTDN AGSVKGRFTI SRDNAKNTVY LQMNSLKPE DTAVYYCAAR AQLMDRSRYS YDYWGQGTQV TVSS |
-Macromolecule #4: ATP-binding cassette sub-family G member 2
| Macromolecule | Name: ATP-binding cassette sub-family G member 2 / type: protein_or_peptide / ID: 4 / Number of copies: 2 / Enantiomer: LEVO / EC number: ABC-type xenobiotic transporter |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 72.385852 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MSSSNVEVFI PVSQGNTNGF PATASNDLKA FTEGAVLSFH NICYRVKLKS GFLPCRKPVE KEILSNINGI MKPGLNAILG PTGGGKSSL LDVLAARKDP SGLSGDVLIN GAPRPANFKC NSGYVVQDDV VMGTLTVREN LQFSAALRLA TTMTNHEKNE R INRVIQEL ...String: MSSSNVEVFI PVSQGNTNGF PATASNDLKA FTEGAVLSFH NICYRVKLKS GFLPCRKPVE KEILSNINGI MKPGLNAILG PTGGGKSSL LDVLAARKDP SGLSGDVLIN GAPRPANFKC NSGYVVQDDV VMGTLTVREN LQFSAALRLA TTMTNHEKNE R INRVIQEL GLDKVADSKV GTQFIRGVSG GERKRTSIGM ELITDPSILF LDEPTTGLDS STANAVLLLL KRMSKQGRTI IF SIHQPRY SIFKLFDSLT LLASGRLMFH GPAQEALGYF ESAGYHCEAY NNPADFFLDI INGDSTAVAL NREEDFKATE IIE PSKQDK PLIEKLAEIY VNSSFYKETK AELHQLSGGE KKKKITVFKE ISYTTSFCHQ LRWVSKRSFK NLLGNPQASI AQII VTVVL GLVIGAIYFG LKNDSTGIQN RAGVLFFLTT NQCFSSVSAV ELFVVEKKLF IHEYISGYYR VSSYFLGKLL SDLLP MRML PSIIFTCIVY FMLGLKPKAD AFFVMMFTLM MVAYSASSMA LAIAAGQSVV SVATLLMTIC FVFMMIFSGL LVNLTT IAS WLSWLQYFSI PRYGFTALQH NEFLGQNFCP GLNATGNNPC NYATCTGEEY LVKQGIDLSP WGLWKNHVAL ACMIVIF LT IAYLKLLFLK KYS UniProtKB: Broad substrate specificity ATP-binding cassette transporter ABCG2 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Vitrification | Cryogen name: ETHANE-PROPANE |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 72.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.4 µm / Nominal defocus min: 0.6 µm |
| Sample stage | Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL |
|---|---|
| Output model |  PDB-8p8a: |
 Movie
Movie Controller
Controller









 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)