+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM map of zebrafish cardiac F-actin | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | F-actin / Actin / Cardiac Actin / Filamentous / CONTRACTILE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationembryonic heart tube development / skeletal muscle fiber development / actin cytoskeleton / ATP binding / nucleus / cytoplasm Similarity search - Function | |||||||||
| Biological species |  | |||||||||
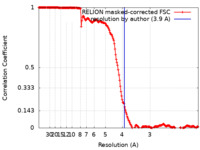
| Method | single particle reconstruction / cryo EM / Resolution: 3.9 Å | |||||||||
 Authors Authors | Bradshaw M / Squire JM / Morris E / Atkinson G / Richardson B / Lees J / Paul DM | |||||||||
| Funding support |  United Kingdom, 1 items United Kingdom, 1 items
| |||||||||
 Citation Citation |  Journal: J Muscle Res Cell Motil / Year: 2023 Journal: J Muscle Res Cell Motil / Year: 2023Title: Zebrafish as a model for cardiac disease; Cryo-EM structure of native cardiac thin filaments from Danio Rerio. Authors: Marston Bradshaw / John M Squire / Edward Morris / Georgia Atkinson / Rebecca Richardson / Jon Lees / Massimo Caputo / Giulia M Bigotti / Danielle M Paul /  Abstract: Actin, tropomyosin and troponin, the proteins that comprise the contractile apparatus of the cardiac thin filament, are highly conserved across species. We have used cryo-EM to study the three- ...Actin, tropomyosin and troponin, the proteins that comprise the contractile apparatus of the cardiac thin filament, are highly conserved across species. We have used cryo-EM to study the three-dimensional structure of the zebrafish cardiac thin and actin filaments. With 70% of human genes having an obvious zebrafish orthologue, and conservation of 85% of disease-causing genes, zebrafish are a good animal model for the study of human disease. Our structure of the zebrafish thin filament reveals the molecular interactions between the constituent proteins, showing that the fundamental organisation of the complex is the same as that reported in the human reconstituted thin filament. A reconstruction of zebrafish cardiac F-actin demonstrates no deviations from human cardiac actin over an extended length of 14 actin subunits. Modelling zebrafish homology models into our maps enabled us to compare, in detail, the similarity with human models. The structural similarities of troponin-T in particular, a region known to contain a hypertrophic cardiomyopathy 'hotspot', confirm the suitability of zebrafish to study these disease-causing mutations. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_17120.map.gz emd_17120.map.gz | 448 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-17120-v30.xml emd-17120-v30.xml emd-17120.xml emd-17120.xml | 19.4 KB 19.4 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_17120_fsc.xml emd_17120_fsc.xml | 17.7 KB | Display |  FSC data file FSC data file |
| Images |  emd_17120.png emd_17120.png | 53.6 KB | ||
| Masks |  emd_17120_msk_1.map emd_17120_msk_1.map | 476.8 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-17120.cif.gz emd-17120.cif.gz | 6.1 KB | ||
| Others |  emd_17120_half_map_1.map.gz emd_17120_half_map_1.map.gz emd_17120_half_map_2.map.gz emd_17120_half_map_2.map.gz | 168.8 MB 168.8 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-17120 http://ftp.pdbj.org/pub/emdb/structures/EMD-17120 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-17120 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-17120 | HTTPS FTP |
-Validation report
| Summary document |  emd_17120_validation.pdf.gz emd_17120_validation.pdf.gz | 955.5 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_17120_full_validation.pdf.gz emd_17120_full_validation.pdf.gz | 955.1 KB | Display | |
| Data in XML |  emd_17120_validation.xml.gz emd_17120_validation.xml.gz | 25.6 KB | Display | |
| Data in CIF |  emd_17120_validation.cif.gz emd_17120_validation.cif.gz | 34.3 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-17120 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-17120 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-17120 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-17120 | HTTPS FTP |
-Related structure data
| Related structure data |  8ordMC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_17120.map.gz / Format: CCP4 / Size: 476.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_17120.map.gz / Format: CCP4 / Size: 476.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.048 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_17120_msk_1.map emd_17120_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_17120_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_17120_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Cardiac F-actin
| Entire | Name: Cardiac F-actin |
|---|---|
| Components |
|
-Supramolecule #1: Cardiac F-actin
| Supramolecule | Name: Cardiac F-actin / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Actin, alpha 1b, skeletal muscle
| Macromolecule | Name: Actin, alpha 1b, skeletal muscle / type: protein_or_peptide / ID: 1 / Number of copies: 5 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 42.021887 KDa |
| Sequence | String: MCDEEETTAL VCDNGSGLVK AGFAGDDAPR AVFPSIVGRP RHQGVMVGMG QKDSYVGDEA QSKRGILTLK YPIEHGIITN WDDMEKIWH HTFYNELRVA PEEHPVLLTE APLNPKANRE KMTQIMFETF NVPAMYVAIQ AVLSLYASGR TTGIVLDAGD G VTHNVPVY ...String: MCDEEETTAL VCDNGSGLVK AGFAGDDAPR AVFPSIVGRP RHQGVMVGMG QKDSYVGDEA QSKRGILTLK YPIEHGIITN WDDMEKIWH HTFYNELRVA PEEHPVLLTE APLNPKANRE KMTQIMFETF NVPAMYVAIQ AVLSLYASGR TTGIVLDAGD G VTHNVPVY EGYALPHAIM RLDLAGRDLT DYLMKILTER GYSFVTTAER EIVRDIKEKL CYVALDFENE MATAASSSSL EK SYELPDG QVITIGNERF RCPETLFQPS FIGMESAGIH ETTYNGIMKC DIDIRKDLYA NNVLSGGTTM YPGIGDRMQK EIT ALAPST MKIKMIAPPE RKYSVWIGGS ILASLSTFQQ MWISKDEYEE AGPSIVHRKC F UniProtKB: Actin alpha 1, skeletal muscle b |
-Macromolecule #2: ADENOSINE-5'-DIPHOSPHATE
| Macromolecule | Name: ADENOSINE-5'-DIPHOSPHATE / type: ligand / ID: 2 / Number of copies: 5 / Formula: ADP |
|---|---|
| Molecular weight | Theoretical: 427.201 Da |
| Chemical component information |  ChemComp-ADP: |
-Macromolecule #3: PHOSPHATE ION
| Macromolecule | Name: PHOSPHATE ION / type: ligand / ID: 3 / Number of copies: 5 / Formula: PO4 |
|---|---|
| Molecular weight | Theoretical: 94.971 Da |
| Chemical component information |  ChemComp-PO4: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | filament |
- Sample preparation
Sample preparation
| Concentration | 0.8 mg/mL | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 6.8 Component:
Details: Phosphate Buffer Protease Inhibitor Cocktail (0.44 mg/ml) | ||||||||||||||||||
| Grid | Model: Quantifoil R2/1 / Material: COPPER / Mesh: 300 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 60 sec. / Pretreatment - Atmosphere: AIR / Pretreatment - Pressure: 0.01 kPa / Details: 15 mAh | ||||||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: LEICA EM GP | ||||||||||||||||||
| Details | Freshly excised heart tissue from Zebrafish. Monodispersed filaments of varying lengths. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Phase plate: VOLTA PHASE PLATE |
| Image recording | Film or detector model: GATAN K2 QUANTUM (4k x 4k) / Detector mode: COUNTING / Digitization - Dimensions - Width: 4096 pixel / Digitization - Dimensions - Height: 4096 pixel / Digitization - Frames/image: 1-35 / Number grids imaged: 2 / Number real images: 5622 / Average electron dose: 38.85 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Calibrated defocus max: 1.0 µm / Calibrated defocus min: 0.5 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 0.9 µm / Nominal defocus min: 0.4 µm / Nominal magnification: 81000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller








 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)