[English] 日本語
 Yorodumi
Yorodumi- EMDB-17110: CryoEM structure of human rho1 GABAA receptor in complex with GAB... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | CryoEM structure of human rho1 GABAA receptor in complex with GABA and picrotoxin | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | GABAA receptor / rho1 / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationGABA receptor activation / GABA-A receptor activity / GABA-gated chloride ion channel activity / GABA-A receptor complex / gamma-aminobutyric acid signaling pathway / intracellular vesicle / chloride channel complex / ligand-gated monoatomic ion channel activity involved in regulation of presynaptic membrane potential / chloride transmembrane transport / modulation of chemical synaptic transmission ...GABA receptor activation / GABA-A receptor activity / GABA-gated chloride ion channel activity / GABA-A receptor complex / gamma-aminobutyric acid signaling pathway / intracellular vesicle / chloride channel complex / ligand-gated monoatomic ion channel activity involved in regulation of presynaptic membrane potential / chloride transmembrane transport / modulation of chemical synaptic transmission / GABA-ergic synapse / presynaptic membrane / chemical synaptic transmission / postsynaptic membrane / protein domain specific binding / protein-containing complex binding / glutamatergic synapse / identical protein binding / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
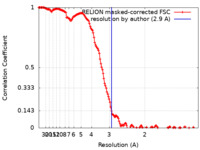
| Method | single particle reconstruction / cryo EM / Resolution: 2.9 Å | |||||||||
 Authors Authors | Chen F / Victor T / John C / Rebecca JH / Erik L | |||||||||
| Funding support |  Sweden, 1 items Sweden, 1 items
| |||||||||
 Citation Citation |  Journal: Neuron / Year: 2023 Journal: Neuron / Year: 2023Title: Structure and dynamics of differential ligand binding in the human ρ-type GABA receptor. Authors: John Cowgill / Chen Fan / Nandan Haloi / Victor Tobiasson / Yuxuan Zhuang / Rebecca J Howard / Erik Lindahl /  Abstract: The neurotransmitter γ-aminobutyric acid (GABA) drives critical inhibitory processes in and beyond the nervous system, partly via ionotropic type-A receptors (GABARs). Pharmacological properties of ...The neurotransmitter γ-aminobutyric acid (GABA) drives critical inhibitory processes in and beyond the nervous system, partly via ionotropic type-A receptors (GABARs). Pharmacological properties of ρ-type GABARs are particularly distinctive, yet the structural basis for their specialization remains unclear. Here, we present cryo-EM structures of a lipid-embedded human ρ1 GABAR, including a partial intracellular domain, under apo, inhibited, and desensitized conditions. An apparent resting state, determined first in the absence of modulators, was recapitulated with the specific inhibitor (1,2,5,6-tetrahydropyridin-4-yl)methylphosphinic acid and blocker picrotoxin and provided a rationale for bicuculline insensitivity. Comparative structures, mutant recordings, and molecular simulations with and without GABA further explained the sensitized but slower activation of ρ1 relative to canonical subtypes. Combining GABA with picrotoxin also captured an apparent uncoupled intermediate state. This work reveals structural mechanisms of gating and modulation with applications to ρ-specific pharmaceutical design and to our biophysical understanding of ligand-gated ion channels. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_17110.map.gz emd_17110.map.gz | 19.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-17110-v30.xml emd-17110-v30.xml emd-17110.xml emd-17110.xml | 20.8 KB 20.8 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_17110_fsc.xml emd_17110_fsc.xml | 15 KB | Display |  FSC data file FSC data file |
| Images |  emd_17110.png emd_17110.png | 226.8 KB | ||
| Filedesc metadata |  emd-17110.cif.gz emd-17110.cif.gz | 6.8 KB | ||
| Others |  emd_17110_half_map_1.map.gz emd_17110_half_map_1.map.gz emd_17110_half_map_2.map.gz emd_17110_half_map_2.map.gz | 226 MB 226 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-17110 http://ftp.pdbj.org/pub/emdb/structures/EMD-17110 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-17110 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-17110 | HTTPS FTP |
-Related structure data
| Related structure data |  8oqaMC  8op9C  8oq6C  8oq7C  8oq8C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_17110.map.gz / Format: CCP4 / Size: 282.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_17110.map.gz / Format: CCP4 / Size: 282.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.6645 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_17110_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_17110_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : human rho1 GABAA receptor
| Entire | Name: human rho1 GABAA receptor |
|---|---|
| Components |
|
-Supramolecule #1: human rho1 GABAA receptor
| Supramolecule | Name: human rho1 GABAA receptor / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Gamma-aminobutyric acid receptor subunit rho-1
| Macromolecule | Name: Gamma-aminobutyric acid receptor subunit rho-1 / type: protein_or_peptide / ID: 1 / Number of copies: 5 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 55.95023 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MLAVPNMRFG IFLLWWGWVL ATESRMHWPG REVHEMSKKG RPQRQRREVH EDAHKQVSPI LRRSPDITKS PLTKSEQLLR IDDHDFSMR PGFGGPAIPV GVDVQVESLD SISEVDMDFT MTLYLRHYWK DERLSFPSTN NLSMTFDGRL VKKIWVPDMF F VHSKRSFI ...String: MLAVPNMRFG IFLLWWGWVL ATESRMHWPG REVHEMSKKG RPQRQRREVH EDAHKQVSPI LRRSPDITKS PLTKSEQLLR IDDHDFSMR PGFGGPAIPV GVDVQVESLD SISEVDMDFT MTLYLRHYWK DERLSFPSTN NLSMTFDGRL VKKIWVPDMF F VHSKRSFI HDTTTDNVML RVQPDGKVLY SLRVTVTAMC NMDFSRFPLD TQTCSLEIES YAYTEDDLML YWKKGNDSLK TD ERISLSQ FLIQEFHTTT KLAFYSSTGW YNRLYINFTL RRHIFFFLLQ TYFPATLMVM LSWVSFWIDR RAVPARVPLG ITT VLTMST IITGVNASMP RVSYIKAVDI YLWVSFVFVF LSVLEYAAVN YLTTVQERKE QKLREKLPCT SGLPPPRTAM LDGN YSDGE VNDLDNYMPE NGEKPDRMMV QLTLASERSS PQRKSQRSSY VSMRIDTHAI DKYSRIIFPA AYILFNLIYW SIFS UniProtKB: Gamma-aminobutyric acid receptor subunit rho-1 |
-Macromolecule #2: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 2 / Number of copies: 10 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Macromolecule #3: HEXANE
| Macromolecule | Name: HEXANE / type: ligand / ID: 3 / Number of copies: 14 / Formula: HEX |
|---|---|
| Molecular weight | Theoretical: 86.175 Da |
| Chemical component information | 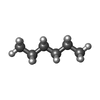 ChemComp-HEX: |
-Macromolecule #4: N-OCTANE
| Macromolecule | Name: N-OCTANE / type: ligand / ID: 4 / Number of copies: 4 / Formula: OCT |
|---|---|
| Molecular weight | Theoretical: 114.229 Da |
| Chemical component information | 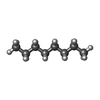 ChemComp-OCT: |
-Macromolecule #5: CHLORIDE ION
| Macromolecule | Name: CHLORIDE ION / type: ligand / ID: 5 / Number of copies: 2 / Formula: CL |
|---|---|
| Molecular weight | Theoretical: 35.453 Da |
-Macromolecule #6: GAMMA-AMINO-BUTANOIC ACID
| Macromolecule | Name: GAMMA-AMINO-BUTANOIC ACID / type: ligand / ID: 6 / Number of copies: 5 / Formula: ABU |
|---|---|
| Molecular weight | Theoretical: 103.12 Da |
| Chemical component information |  ChemComp-ABU: |
-Macromolecule #7: (1aR,2aR,3S,6R,6aS,8aS,8bR,9R)-2a-hydroxy-8b-methyl-9-(prop-1-en-...
| Macromolecule | Name: (1aR,2aR,3S,6R,6aS,8aS,8bR,9R)-2a-hydroxy-8b-methyl-9-(prop-1-en-2-yl)hexahydro-3,6-methano-1,5,7-trioxacyclopenta[ij]c yclopropa[a]azulene-4,8(3H)-dione type: ligand / ID: 7 / Number of copies: 1 / Formula: RI5 |
|---|---|
| Molecular weight | Theoretical: 292.284 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 298 K |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 46.12 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.8000000000000003 µm / Nominal defocus min: 1.2 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller








 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)