+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM captures early ribosome assembly in action | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | ribosome / ribosome assembly / ribosome biogenesis / total reconstitution / RNA / ribosomal protein. | |||||||||
| Function / homology |  Function and homology information Function and homology informationtranscriptional attenuation / endoribonuclease inhibitor activity / RNA-binding transcription regulator activity / positive regulation of ribosome biogenesis / negative regulation of cytoplasmic translation / DnaA-L2 complex / translation repressor activity / negative regulation of DNA-templated DNA replication initiation / mRNA regulatory element binding translation repressor activity / ribosome assembly ...transcriptional attenuation / endoribonuclease inhibitor activity / RNA-binding transcription regulator activity / positive regulation of ribosome biogenesis / negative regulation of cytoplasmic translation / DnaA-L2 complex / translation repressor activity / negative regulation of DNA-templated DNA replication initiation / mRNA regulatory element binding translation repressor activity / ribosome assembly / assembly of large subunit precursor of preribosome / cytosolic ribosome assembly / response to reactive oxygen species / regulation of cell growth / DNA-templated transcription termination / response to radiation / mRNA 5'-UTR binding / large ribosomal subunit / ribosome binding / 5S rRNA binding / large ribosomal subunit rRNA binding / transferase activity / ribosomal large subunit assembly / cytoplasmic translation / cytosolic large ribosomal subunit / tRNA binding / negative regulation of translation / rRNA binding / ribosome / structural constituent of ribosome / translation / response to antibiotic / negative regulation of DNA-templated transcription / mRNA binding / DNA binding / RNA binding / zinc ion binding / cytosol / cytoplasm Similarity search - Function | |||||||||
| Biological species |  | |||||||||
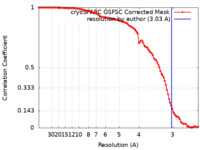
| Method | single particle reconstruction / cryo EM / Resolution: 3.03 Å | |||||||||
 Authors Authors | Lauer S / Nikolay R / Qin B | |||||||||
| Funding support |  Germany, 1 items Germany, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: Cryo-EM captures early ribosome assembly in action. Authors: Bo Qin / Simon M Lauer / Annika Balke / Carlos H Vieira-Vieira / Jörg Bürger / Thorsten Mielke / Matthias Selbach / Patrick Scheerer / Christian M T Spahn / Rainer Nikolay /  Abstract: Ribosome biogenesis is a fundamental multi-step cellular process in all domains of life that involves the production, processing, folding, and modification of ribosomal RNAs (rRNAs) and ribosomal ...Ribosome biogenesis is a fundamental multi-step cellular process in all domains of life that involves the production, processing, folding, and modification of ribosomal RNAs (rRNAs) and ribosomal proteins. To obtain insights into the still unexplored early assembly phase of the bacterial 50S subunit, we exploited a minimal in vitro reconstitution system using purified ribosomal components and scalable reaction conditions. Time-limited assembly assays combined with cryo-EM analysis visualizes the structurally complex assembly pathway starting with a particle consisting of ordered density for only ~500 nucleotides of 23S rRNA domain I and three ribosomal proteins. In addition, our structural analysis reveals that early 50S assembly occurs in a domain-wise fashion, while late 50S assembly proceeds incrementally. Furthermore, we find that both ribosomal proteins and folded rRNA helices, occupying surface exposed regions on pre-50S particles, induce, or stabilize rRNA folds within adjacent regions, thereby creating cooperativity. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_16495.map.gz emd_16495.map.gz | 97.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-16495-v30.xml emd-16495-v30.xml emd-16495.xml emd-16495.xml | 43.2 KB 43.2 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_16495_fsc.xml emd_16495_fsc.xml | 9.4 KB | Display |  FSC data file FSC data file |
| Images |  emd_16495.png emd_16495.png | 109.7 KB | ||
| Masks |  emd_16495_msk_1.map emd_16495_msk_1.map | 103 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-16495.cif.gz emd-16495.cif.gz | 9.7 KB | ||
| Others |  emd_16495_half_map_1.map.gz emd_16495_half_map_1.map.gz emd_16495_half_map_2.map.gz emd_16495_half_map_2.map.gz | 95.6 MB 95.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-16495 http://ftp.pdbj.org/pub/emdb/structures/EMD-16495 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-16495 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-16495 | HTTPS FTP |
-Validation report
| Summary document |  emd_16495_validation.pdf.gz emd_16495_validation.pdf.gz | 1.1 MB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_16495_full_validation.pdf.gz emd_16495_full_validation.pdf.gz | 1.1 MB | Display | |
| Data in XML |  emd_16495_validation.xml.gz emd_16495_validation.xml.gz | 17 KB | Display | |
| Data in CIF |  emd_16495_validation.cif.gz emd_16495_validation.cif.gz | 22.8 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-16495 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-16495 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-16495 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-16495 | HTTPS FTP |
-Related structure data
| Related structure data |  8c8yMC  8c8xC  8c8zC  8c90C  8c91C  8c92C  8c93C  8c94C  8c95C  8c96C  8c97C  8c98C  8c99C  8c9aC  8c9bC  8c9cC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_16495.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_16495.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.25 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_16495_msk_1.map emd_16495_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_16495_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_16495_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
+Entire : large ribosomal subunit precursor C-CP_L2/L28
+Supramolecule #1: large ribosomal subunit precursor C-CP_L2/L28
+Macromolecule #1: 50S ribosomal protein L25
+Macromolecule #3: 50S ribosomal protein L2
+Macromolecule #4: 50S ribosomal protein L3
+Macromolecule #5: 50S ribosomal protein L4
+Macromolecule #6: 50S ribosomal protein L13
+Macromolecule #7: 50S ribosomal protein L14
+Macromolecule #8: 50S ribosomal protein L15
+Macromolecule #9: 50S ribosomal protein L17
+Macromolecule #10: 50S ribosomal protein L19
+Macromolecule #11: 50S ribosomal protein L20
+Macromolecule #12: 50S ribosomal protein L21
+Macromolecule #13: 50S ribosomal protein L22
+Macromolecule #14: 50S ribosomal protein L23
+Macromolecule #15: 50S ribosomal protein L24
+Macromolecule #16: 50S ribosomal protein L28
+Macromolecule #17: 50S ribosomal protein L29
+Macromolecule #18: 50S ribosomal protein L32
+Macromolecule #19: 50S ribosomal protein L34
+Macromolecule #20: 50S ribosomal protein L18
+Macromolecule #21: 50S ribosomal protein L5
+Macromolecule #23: 50S ribosomal protein L30
+Macromolecule #24: 50S ribosomal protein L9
+Macromolecule #25: 50S ribosomal protein L27
+Macromolecule #2: 23S rRNA
+Macromolecule #22: 5S rRNA
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.6 |
|---|---|
| Grid | Model: Quantifoil / Material: COPPER / Mesh: 400 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 20 sec. |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI POLARA 300 |
|---|---|
| Temperature | Min: 82.0 K / Max: 83.0 K |
| Software | Name: Leginon |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Average exposure time: 10.0 sec. / Average electron dose: 62.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: OTHER / Cs: 2.0 mm / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.5 µm / Nominal magnification: 31000 |
| Sample stage | Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Tecnai Polara / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Software | Name:  Coot (ver. 0.9.6.2) Coot (ver. 0.9.6.2) |
|---|---|
| Refinement | Space: REAL |
| Output model |  PDB-8c8y: |
 Movie
Movie Controller
Controller























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)