[English] 日本語
 Yorodumi
Yorodumi- EMDB-16354: Structure of SLC40/ferroportin in complex with vamifeport and syn... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of SLC40/ferroportin in complex with vamifeport and synthetic nanobody Sy12 in outward-facing conformation | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Iron / Inhibitor / SLC40 / Transporter / TRANSPORT PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationspleen trabecula formation / iron ion export across plasma membrane / Defective SLC40A1 causes hemochromatosis 4 (HFE4) (duodenum) / Defective SLC40A1 causes hemochromatosis 4 (HFE4) (macrophages) / Defective CP causes aceruloplasminemia (ACERULOP) / Metal ion SLC transporters / lymphocyte homeostasis / ferrous iron transmembrane transporter activity / endothelium development / iron ion transmembrane transporter activity ...spleen trabecula formation / iron ion export across plasma membrane / Defective SLC40A1 causes hemochromatosis 4 (HFE4) (duodenum) / Defective SLC40A1 causes hemochromatosis 4 (HFE4) (macrophages) / Defective CP causes aceruloplasminemia (ACERULOP) / Metal ion SLC transporters / lymphocyte homeostasis / ferrous iron transmembrane transporter activity / endothelium development / iron ion transmembrane transporter activity / iron ion transmembrane transport / peptide hormone binding / establishment of localization in cell / Iron uptake and transport / multicellular organismal-level iron ion homeostasis / synaptic vesicle / basolateral plasma membrane / intracellular iron ion homeostasis / transcription by RNA polymerase II / apoptotic process / negative regulation of apoptotic process / positive regulation of transcription by RNA polymerase II / nucleoplasm / metal ion binding / identical protein binding / membrane / plasma membrane / cytosol / cytoplasm Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
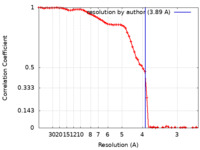
| Method | single particle reconstruction / cryo EM / Resolution: 3.89 Å | |||||||||
 Authors Authors | Lehmann EF / Liziczai M / Drozdzyk K / Dutzler R / Manatschal C | |||||||||
| Funding support |  Switzerland, 1 items Switzerland, 1 items
| |||||||||
 Citation Citation |  Journal: Elife / Year: 2023 Journal: Elife / Year: 2023Title: Structures of ferroportin in complex with its specific inhibitor vamifeport. Authors: Elena Farah Lehmann / Márton Liziczai / Katarzyna Drożdżyk / Patrick Altermatt / Cassiano Langini / Vania Manolova / Hanna Sundstrom / Franz Dürrenberger / Raimund Dutzler / Cristina Manatschal /  Abstract: A central regulatory mechanism of iron homeostasis in humans involves ferroportin (FPN), the sole cellular iron exporter, and the peptide hormone hepcidin, which inhibits Fe transport and induces ...A central regulatory mechanism of iron homeostasis in humans involves ferroportin (FPN), the sole cellular iron exporter, and the peptide hormone hepcidin, which inhibits Fe transport and induces internalization and degradation of FPN. Dysregulation of the FPN/hepcidin axis leads to diverse pathological conditions, and consequently, pharmacological compounds that inhibit FPN-mediated iron transport are of high clinical interest. Here, we describe the cryo-electron microscopy structures of human FPN in complex with synthetic nanobodies and vamifeport (VIT-2763), the first clinical-stage oral FPN inhibitor. Vamifeport competes with hepcidin for FPN binding and is currently in clinical development for β-thalassemia and sickle cell disease. The structures display two distinct conformations of FPN, representing outward-facing and occluded states of the transporter. The vamifeport site is located in the center of the protein, where the overlap with hepcidin interactions underlies the competitive relationship between the two molecules. The introduction of point mutations in the binding pocket of vamifeport reduces its affinity to FPN, emphasizing the relevance of the structural data. Together, our study reveals conformational rearrangements of FPN that are of potential relevance for transport, and it provides initial insight into the pharmacological targeting of this unique iron efflux transporter. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_16354.map.gz emd_16354.map.gz | 21 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-16354-v30.xml emd-16354-v30.xml emd-16354.xml emd-16354.xml | 14.5 KB 14.5 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_16354_fsc.xml emd_16354_fsc.xml | 8.3 KB | Display |  FSC data file FSC data file |
| Images |  emd_16354.png emd_16354.png | 60.1 KB | ||
| Masks |  emd_16354_msk_1.map emd_16354_msk_1.map | 22.2 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-16354.cif.gz emd-16354.cif.gz | 5.7 KB | ||
| Others |  emd_16354_half_map_1.map.gz emd_16354_half_map_1.map.gz emd_16354_half_map_2.map.gz emd_16354_half_map_2.map.gz | 20.7 MB 20.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-16354 http://ftp.pdbj.org/pub/emdb/structures/EMD-16354 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-16354 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-16354 | HTTPS FTP |
-Related structure data
| Related structure data |  8c03MC  8bzyC  8c02C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_16354.map.gz / Format: CCP4 / Size: 22.2 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_16354.map.gz / Format: CCP4 / Size: 22.2 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.302 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_16354_msk_1.map emd_16354_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_16354_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_16354_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Complex of ferroportin with synthetic nanobody and inhibitor
| Entire | Name: Complex of ferroportin with synthetic nanobody and inhibitor |
|---|---|
| Components |
|
-Supramolecule #1: Complex of ferroportin with synthetic nanobody and inhibitor
| Supramolecule | Name: Complex of ferroportin with synthetic nanobody and inhibitor type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 Details: Ferroportin was expressed in human derived HEK cells, whereas the synthetic nanobody was expressed in bacterial cell culture. The two were purified separately and mixed shortly before vitrification |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Solute carrier family 40 member 1
| Macromolecule | Name: Solute carrier family 40 member 1 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 63.536848 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MSTRAGDHNR QRGCCGSLAD YLTSAKFLLY LGHSLSTWGD RMWHFAVSVF LVELYGNSLL LTAVYGLVVA GSVLVLGAII GDWVDKNAR LKVAQTSLVV QNVSVILCGI ILMMVFLHKH ELLTMYHGWV LTSCYILIIT IANIANLAST ATAITIQRDW I VVVAGEDR ...String: MSTRAGDHNR QRGCCGSLAD YLTSAKFLLY LGHSLSTWGD RMWHFAVSVF LVELYGNSLL LTAVYGLVVA GSVLVLGAII GDWVDKNAR LKVAQTSLVV QNVSVILCGI ILMMVFLHKH ELLTMYHGWV LTSCYILIIT IANIANLAST ATAITIQRDW I VVVAGEDR SKLANMNATI RRIDQLTNIL APMAVGQIMT FGSPVIGCGF ISGWNLVSMC VEYVLLWKVY QKTPALAVKA GL KEEETEL KQLNLHKDTE PKPLEGTHLM GVKDSNIHEL EHEQEPTCAS QMAEPFRTFR DGWVSYYNQP VFLAGMGLAF LYM TVLGFD CITTGYAYTQ GLSGSILSIL MGASAITGIM GTVAFTWLRR KCGLVRTGLI SGLAQLSCLI LCVISVFMPG SPLD LSVSP FEDIRSRFIQ GESITPTKIP EITTEIYMSN GSNSANIVPE TSPESVPIIS VSLLFAGVIA ARIGLWSFDL TVTQL LQEN VIESERGIIN GVQNSMNYLL DLLHFIMVIL APNPEAFGLL VLISVSFVAM GHIMYFRFAQ NTLGNKLFAC GPDAKE VRK ENQANTSVVA LEVLFQG UniProtKB: Ferroportin |
-Macromolecule #2: 2-[2-[2-(1~{H}-benzimidazol-2-yl)ethylamino]ethyl]-~{N}-[(3-fluor...
| Macromolecule | Name: 2-[2-[2-(1~{H}-benzimidazol-2-yl)ethylamino]ethyl]-~{N}-[(3-fluoranylpyridin-2-yl)methyl]-1,3-oxazole-4-carboxamide type: ligand / ID: 2 / Number of copies: 1 / Formula: SZU |
|---|---|
| Molecular weight | Theoretical: 408.429 Da |
| Chemical component information | 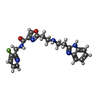 ChemComp-SZU: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE-PROPANE / Chamber humidity: 100 % / Chamber temperature: 278 K |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 70.2 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.4 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller








 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)