登録情報 データベース : EMDB / ID : EMD-16312タイトル In situ outer dynein arm from Chlamydomonas reinhardtii in the post-power stroke state 細胞器官・細胞要素 : In situ outer dynein armリガンド : x 3種 / / / / 機能・相同性 分子機能 ドメイン・相同性 構成要素
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / 生物種 Chlamydomonas reinhardtii (クラミドモナス) / Tetrahymena thermophila (真核生物)手法 / / 解像度 : 30.3 Å Zimmermann NEL / Noga A / Obbineni JM / Ishikawa T 資金援助 Organization Grant number 国 Swiss National Science Foundation NF310030_192644
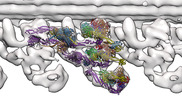
ジャーナル : EMBO J / 年 : 2023タイトル : ATP-induced conformational change of axonemal outer dynein arms revealed by cryo-electron tomography.著者 : Noemi Zimmermann / Akira Noga / Jagan Mohan Obbineni / Takashi Ishikawa / 要旨 : Axonemal outer dynein arm (ODA) motors generate force for ciliary beating. We analyzed three states of the ODA during the power stroke cycle using in situ cryo-electron tomography, subtomogram ... Axonemal outer dynein arm (ODA) motors generate force for ciliary beating. We analyzed three states of the ODA during the power stroke cycle using in situ cryo-electron tomography, subtomogram averaging, and classification. These states of force generation depict the prepower stroke, postpower stroke, and intermediate state conformations. Comparison of these conformations to published in vitro atomic structures of cytoplasmic dynein, ODA, and the Shulin-ODA complex revealed differences in the orientation and position of the dynein head. Our analysis shows that in the absence of ATP, all dynein linkers interact with the AAA3/AAA4 domains, indicating that interactions with the adjacent microtubule doublet B-tubule direct dynein orientation. For the prepower stroke conformation, there were changes in the tail that is anchored on the A-tubule. We built models starting with available high-resolution structures to generate a best-fitting model structure for the in situ pre- and postpower stroke ODA conformations, thereby showing that ODA in a complex with Shulin adopts a similar conformation as the active prepower stroke ODA in the axoneme. 履歴 登録 2022年12月8日 - ヘッダ(付随情報) 公開 2023年5月10日 - マップ公開 2023年5月10日 - 更新 2025年10月1日 - 現状 2025年10月1日 処理サイト : PDBe / 状態 : 公開
すべて表示 表示を減らす
 データを開く
データを開く 基本情報
基本情報
 マップデータ
マップデータ 試料
試料 キーワード
キーワード 機能・相同性情報
機能・相同性情報

 データ登録者
データ登録者 スイス, 1件
スイス, 1件  引用
引用 ジャーナル: EMBO J / 年: 2023
ジャーナル: EMBO J / 年: 2023

 構造の表示
構造の表示 ダウンロードとリンク
ダウンロードとリンク emd_16312.map.gz
emd_16312.map.gz EMDBマップデータ形式
EMDBマップデータ形式 emd-16312-v30.xml
emd-16312-v30.xml emd-16312.xml
emd-16312.xml EMDBヘッダ
EMDBヘッダ emd_16312.png
emd_16312.png emd-16312.cif.gz
emd-16312.cif.gz emd_16312_half_map_1.map.gz
emd_16312_half_map_1.map.gz emd_16312_half_map_2.map.gz
emd_16312_half_map_2.map.gz http://ftp.pdbj.org/pub/emdb/structures/EMD-16312
http://ftp.pdbj.org/pub/emdb/structures/EMD-16312 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-16312
ftp://ftp.pdbj.org/pub/emdb/structures/EMD-16312 emd_16312_validation.pdf.gz
emd_16312_validation.pdf.gz EMDB検証レポート
EMDB検証レポート emd_16312_full_validation.pdf.gz
emd_16312_full_validation.pdf.gz emd_16312_validation.xml.gz
emd_16312_validation.xml.gz emd_16312_validation.cif.gz
emd_16312_validation.cif.gz https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-16312
https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-16312 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-16312
ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-16312 リンク
リンク EMDB (EBI/PDBe) /
EMDB (EBI/PDBe) /  EMDataResource
EMDataResource マップ
マップ ダウンロード / ファイル: emd_16312.map.gz / 形式: CCP4 / 大きさ: 1.5 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES)
ダウンロード / ファイル: emd_16312.map.gz / 形式: CCP4 / 大きさ: 1.5 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) 試料の構成要素
試料の構成要素 解析
解析 試料調製
試料調製 電子顕微鏡法
電子顕微鏡法 FIELD EMISSION GUN
FIELD EMISSION GUN
 画像解析
画像解析
 ムービー
ムービー コントローラー
コントローラー
















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)