[English] 日本語
 Yorodumi
Yorodumi- EMDB-16137: BAM-EspP complex structure with BamA-G431C/EspP-N1293C mutations ... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | BAM-EspP complex structure with BamA-G431C/EspP-N1293C mutations in nanodisc | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | BAM / BamABCDE / EspP / Gram-negative bacteria / outer membrane protein / outer membrane barrel / BamA / BamB / BamC / BamD / BamE / MEMBRANE PROTEIN / Outer membrane protein insertion and release / TRANSPORT PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationBam protein complex / Gram-negative-bacterium-type cell outer membrane assembly / Secretion of toxins / protein insertion into membrane / Hydrolases; Acting on peptide bonds (peptidases); Serine endopeptidases / cell outer membrane / protein-macromolecule adaptor activity / periplasmic space / cell adhesion / serine-type endopeptidase activity ...Bam protein complex / Gram-negative-bacterium-type cell outer membrane assembly / Secretion of toxins / protein insertion into membrane / Hydrolases; Acting on peptide bonds (peptidases); Serine endopeptidases / cell outer membrane / protein-macromolecule adaptor activity / periplasmic space / cell adhesion / serine-type endopeptidase activity / response to antibiotic / cell surface / proteolysis / extracellular region / identical protein binding / membrane Similarity search - Function | |||||||||
| Biological species |   | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.5 Å | |||||||||
 Authors Authors | Shen C / Chang S / Luo Q / Zhang Z / Xie T / Luo B / Lu G / Zhu X / Wei X / Dong C ...Shen C / Chang S / Luo Q / Zhang Z / Xie T / Luo B / Lu G / Zhu X / Wei X / Dong C / Zhou R / Zhang X / Tang X / Dong H | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2023 Journal: Nature / Year: 2023Title: Structural basis of BAM-mediated outer membrane β-barrel protein assembly. Authors: Chongrong Shen / Shenghai Chang / Qinghua Luo / Kevin Chun Chan / Zhibo Zhang / Bingnan Luo / Teng Xie / Guangwen Lu / Xiaofeng Zhu / Xiawei Wei / Changjiang Dong / Ruhong Zhou / Xing Zhang ...Authors: Chongrong Shen / Shenghai Chang / Qinghua Luo / Kevin Chun Chan / Zhibo Zhang / Bingnan Luo / Teng Xie / Guangwen Lu / Xiaofeng Zhu / Xiawei Wei / Changjiang Dong / Ruhong Zhou / Xing Zhang / Xiaodi Tang / Haohao Dong /   Abstract: The outer membrane structure is common in Gram-negative bacteria, mitochondria and chloroplasts, and contains outer membrane β-barrel proteins (OMPs) that are essential interchange portals of ...The outer membrane structure is common in Gram-negative bacteria, mitochondria and chloroplasts, and contains outer membrane β-barrel proteins (OMPs) that are essential interchange portals of materials. All known OMPs share the antiparallel β-strand topology, implicating a common evolutionary origin and conserved folding mechanism. Models have been proposed for bacterial β-barrel assembly machinery (BAM) to initiate OMP folding; however, mechanisms by which BAM proceeds to complete OMP assembly remain unclear. Here we report intermediate structures of BAM assembling an OMP substrate, EspP, demonstrating sequential conformational dynamics of BAM during the late stages of OMP assembly, which is further supported by molecular dynamics simulations. Mutagenic in vitro and in vivo assembly assays reveal functional residues of BamA and EspP for barrel hybridization, closure and release. Our work provides novel insights into the common mechanism of OMP assembly. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_16137.map.gz emd_16137.map.gz | 33 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-16137-v30.xml emd-16137-v30.xml emd-16137.xml emd-16137.xml | 25.7 KB 25.7 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_16137.png emd_16137.png | 102 KB | ||
| Filedesc metadata |  emd-16137.cif.gz emd-16137.cif.gz | 7.5 KB | ||
| Others |  emd_16137_additional_1.map.gz emd_16137_additional_1.map.gz emd_16137_half_map_1.map.gz emd_16137_half_map_1.map.gz emd_16137_half_map_2.map.gz emd_16137_half_map_2.map.gz | 32.9 MB 59.3 MB 59.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-16137 http://ftp.pdbj.org/pub/emdb/structures/EMD-16137 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-16137 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-16137 | HTTPS FTP |
-Related structure data
| Related structure data |  8bnzMC  7ye4C  7ye6C  8bo2C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_16137.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_16137.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.93 Å | ||||||||||||||||||||||||||||||||||||
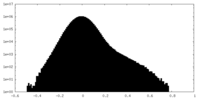
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: local refinement result of the TMD
| File | emd_16137_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | local refinement result of the TMD | ||||||||||||
| Projections & Slices |
| ||||||||||||
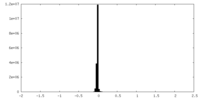
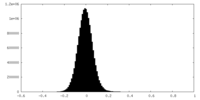
| Density Histograms |
-Half map: #2
| File | emd_16137_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
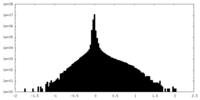
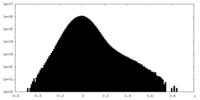
| Density Histograms |
-Half map: #1
| File | emd_16137_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
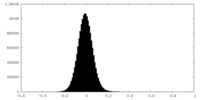
| Density Histograms |
- Sample components
Sample components
-Entire : BAM-EspP complex with BamA-G431C/EspP-N1293C mutations in nanodisc
| Entire | Name: BAM-EspP complex with BamA-G431C/EspP-N1293C mutations in nanodisc |
|---|---|
| Components |
|
-Supramolecule #1: BAM-EspP complex with BamA-G431C/EspP-N1293C mutations in nanodisc
| Supramolecule | Name: BAM-EspP complex with BamA-G431C/EspP-N1293C mutations in nanodisc type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|
-Supramolecule #2: BAM
| Supramolecule | Name: BAM / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1-#5 |
|---|---|
| Source (natural) | Organism:  |
-Supramolecule #3: EspP
| Supramolecule | Name: EspP / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #6 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Outer membrane protein assembly factor BamA
| Macromolecule | Name: Outer membrane protein assembly factor BamA / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 90.689477 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MAMKKLLIAS LLFSSATVYG AEGFVVKDIH FEGLQRVAVG AALLSMPVRT GDTVNDEDIS NTIRALFATG NFEDVRVLRD GDTLLVQVK ERPTIASITF SGNKSVKDDM LKQNLEASGV RVGESLDRTT IADIEKGLED FYYSVGKYSA SVKAVVTPLP R NRVDLKLV ...String: MAMKKLLIAS LLFSSATVYG AEGFVVKDIH FEGLQRVAVG AALLSMPVRT GDTVNDEDIS NTIRALFATG NFEDVRVLRD GDTLLVQVK ERPTIASITF SGNKSVKDDM LKQNLEASGV RVGESLDRTT IADIEKGLED FYYSVGKYSA SVKAVVTPLP R NRVDLKLV FQEGVSAEIQ QINIVGNHAF TTDELISHFQ LRDEVPWWNV VGDRKYQKQK LAGDLETLRS YYLDRGYARF NI DSTQVSL TPDKKGIYVT VNITEGDQYK LSGVEVSGNL AGHSAEIEQL TKIEPGELYN GTKVTKMEDD IKKLLGRYGY AYP RVQSMP EINDADKTVK LRVNVDAGNR FYVRKIRFEG NDTSKDAVLR REMRQMEGAW LGSDLVDQGK ERLNRLGFFE TVDT DTQRV PGSPDQVDVV YKVKERNTGS FNFGICYGTE SGVSFQAGVQ QDNWLGTGYA VGINGTKNDY QTYAELSVTN PYFTV DGVS LGGRLFYNDF QADDADLSDY TNKSYGTDVT LGFPINEYNS LRAGLGYVHN SLSNMQPQVA MWRYLYSMGE HPSTSD QDN SFKTDDFTFN YGWTYNKLDR GYFPTDGSRV NLTGKVTIPG SDNEYYKVTL DTATYVPIDD DHKWVVLGRT RWGYGDG LG GKEMPFYENF YAGGSSTVRG FQSNTIGPKA VYFPHQASNY DPDYDYECAT QDGAKDLCKS DDAVGGNAMA VASLEFIT P TPFISDKYAN SVRTSFFWDM GTVWDTNWDS SQYSGYPDYS DPSNIRMSAG IALQWMSPLG PLVFSYAQPF KKYDGDKAE QFQFNIGKTW UniProtKB: Outer membrane protein assembly factor BamA |
-Macromolecule #2: Outer membrane protein assembly factor BamB
| Macromolecule | Name: Outer membrane protein assembly factor BamB / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 41.918945 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MQLRKLLLPG LLSVTLLSGC SLFNSEEDVV KMSPLPTVEN QFTPTTAWST SVGSGIGNFY SNLHPALADN VVYAADRAGL VKALNADDG KEIWSVSLAE KDGWFSKEPA LLSGGVTVSG GHVYIGSEKA QVYALNTSDG TVAWQTKVAG EALSRPVVSD G LVLIHTSN ...String: MQLRKLLLPG LLSVTLLSGC SLFNSEEDVV KMSPLPTVEN QFTPTTAWST SVGSGIGNFY SNLHPALADN VVYAADRAGL VKALNADDG KEIWSVSLAE KDGWFSKEPA LLSGGVTVSG GHVYIGSEKA QVYALNTSDG TVAWQTKVAG EALSRPVVSD G LVLIHTSN GQLQALNEAD GAVKWTVNLD MPSLSLRGES APTTAFGAAV VGGDNGRVSA VLMEQGQMIW QQRISQATGS TE IDRLSDV DTTPVVVNGV VFALAYNGNL TALDLRSGQI MWKRELGSVN DFIVDGNRIY LVDQNDRVMA LTIDGGVTLW TQS DLLHRL LTSPVLYNGN LVVGDSEGYL HWINVEDGRF VAQQKVDSSG FQTEPVAADG KLLIQAKDGT VYSITR UniProtKB: Outer membrane protein assembly factor BamB |
-Macromolecule #3: Outer membrane protein assembly factor BamC
| Macromolecule | Name: Outer membrane protein assembly factor BamC / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 36.875277 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MAYSVQKSRL AKVAGVSLVL LLAACSSDSR YKRQVSGDEA YLEAAPLAEL HAPAGMILPV TSGDYAIPVT NGSGAVGKAL DIRPPAQPL ALVSGARTQF TGDTASLLVE NGRGNTLWPQ VVSVLQAKNY TITQRDDAGQ TLTTDWVQWN RLDEDEQYRG R YQISVKPQ ...String: MAYSVQKSRL AKVAGVSLVL LLAACSSDSR YKRQVSGDEA YLEAAPLAEL HAPAGMILPV TSGDYAIPVT NGSGAVGKAL DIRPPAQPL ALVSGARTQF TGDTASLLVE NGRGNTLWPQ VVSVLQAKNY TITQRDDAGQ TLTTDWVQWN RLDEDEQYRG R YQISVKPQ GYQQAVTVKL LNLEQAGKPV ADAASMQRYS TEMMNVISAG LDKSATDAAN AAQNRASTTM DVQSAADDTG LP MLVVRGP FNVVWQRLPA ALEKVGMKVT DSTRSQGNMA VTYKPLSDSD WQELGASDPG LASGDYKLQV GDLDNRSSLQ FID PKGHTL TQSQNDALVA VFQAAFSK UniProtKB: Outer membrane protein assembly factor BamC |
-Macromolecule #4: Outer membrane protein assembly factor BamD
| Macromolecule | Name: Outer membrane protein assembly factor BamD / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 27.85835 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MTRMKYLVAA ATLSLFLAGC SGSKEEVPDN PPNEIYATAQ QKLQDGNWRQ AITQLEALDN RYPFGPYSQQ VQLDLIYAYY KNADLPLAQ AAIDRFIRLN PTHPNIDYVM YMRGLTNMAL DDSALQGFFG VDRSDRDPQH ARAAFSDFSK LVRGYPNSQY T TDATKRLV ...String: MTRMKYLVAA ATLSLFLAGC SGSKEEVPDN PPNEIYATAQ QKLQDGNWRQ AITQLEALDN RYPFGPYSQQ VQLDLIYAYY KNADLPLAQ AAIDRFIRLN PTHPNIDYVM YMRGLTNMAL DDSALQGFFG VDRSDRDPQH ARAAFSDFSK LVRGYPNSQY T TDATKRLV FLKDRLAKYE YSVAEYYTER GAWVAVVNRV EGMLRDYPDT QATRDALPLM ENAYRQMQMN AQAEKVAKII AA NSSNT UniProtKB: Outer membrane protein assembly factor BamD |
-Macromolecule #5: Outer membrane protein assembly factor BamE
| Macromolecule | Name: Outer membrane protein assembly factor BamE / type: protein_or_peptide / ID: 5 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 13.530256 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MRCKTLTAAA AVLLMLTAGC STLERVVYRP DINQGNYLTA NDVSKIRVGM TQQQVAYALG TPLMSDPFGT NTWFYVFRQQ PGHEGVTQQ TLTLTFNSSG VLTNIDNKPA LSGNGGHHHH HHHH UniProtKB: Outer membrane protein assembly factor BamE |
-Macromolecule #6: Serine protease EspP
| Macromolecule | Name: Serine protease EspP / type: protein_or_peptide / ID: 6 / Number of copies: 1 / Enantiomer: LEVO EC number: Hydrolases; Acting on peptide bonds (peptidases); Serine endopeptidases |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 38.727812 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: NIELVSAPKD TNENVFKASK QTIGFSDVTP VITTRETDDK ITWSLTGYNT VANKEATRNA AALFSVDYKA FLNEVNNLNK RMGDLRDIN GEAGAWARIM SGTGSASGGF SDNYTHVQVG VDKKHELDGL DLFTGFTVTH TDSSASADVF SGKTKSVGAG L YASAMFDS ...String: NIELVSAPKD TNENVFKASK QTIGFSDVTP VITTRETDDK ITWSLTGYNT VANKEATRNA AALFSVDYKA FLNEVNNLNK RMGDLRDIN GEAGAWARIM SGTGSASGGF SDNYTHVQVG VDKKHELDGL DLFTGFTVTH TDSSASADVF SGKTKSVGAG L YASAMFDS GAYIDLIGKY VHHDNEYTAT FAGLGTRDYS THSWYAGAEA GYRYHVTEDA WIEPQAELVY GSVSGKQFAW KD QGMHLSM KDKDYNPLIG RTGVDVGKSF SGKDWKVTAR AGLGYQFDLL ANGETVLRDA SGEKRIKGEK DSRMLMSVGL NAE IRDNVR FGLEFEKSAF GKYNVDNAVC ANFRYSF UniProtKB: Serine protease EspP |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.8 |
|---|---|
| Grid | Model: Quantifoil R1.2/1.3 / Pretreatment - Type: GLOW DISCHARGE |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated defocus max: 2.0 µm / Calibrated defocus min: 1.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: NONE |
|---|---|
| Final reconstruction | Applied symmetry - Point group: C1 (asymmetric) / Resolution.type: BY AUTHOR / Resolution: 3.5 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 170620 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller











 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)