[English] 日本語
 Yorodumi
Yorodumi- EMDB-16019: Sarkosyl-extracted AppNL-G-F Abeta42 fibril structure (Methoxy-X0... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Sarkosyl-extracted AppNL-G-F Abeta42 fibril structure (Methoxy-X04-labelled mice) | |||||||||
 Map data Map data | CryoEM map for extracted AppNLGF Abeta42 fibril after MOX04 labelling | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Amyloid / fibril / helical / cross-beta / beta amyloid / PROTEIN FIBRIL / ex vivo / arctic mutant / alzheimers disease | |||||||||
| Function / homology |  Function and homology information Function and homology informationamyloid-beta complex / growth cone lamellipodium / cellular response to norepinephrine stimulus / growth cone filopodium / microglia development / collateral sprouting in absence of injury / Formyl peptide receptors bind formyl peptides and many other ligands / axo-dendritic transport / regulation of Wnt signaling pathway / hippocampal neuron apoptotic process ...amyloid-beta complex / growth cone lamellipodium / cellular response to norepinephrine stimulus / growth cone filopodium / microglia development / collateral sprouting in absence of injury / Formyl peptide receptors bind formyl peptides and many other ligands / axo-dendritic transport / regulation of Wnt signaling pathway / hippocampal neuron apoptotic process / axon midline choice point recognition / astrocyte activation involved in immune response / regulation of synapse structure or activity / NMDA selective glutamate receptor signaling pathway / regulation of spontaneous synaptic transmission / mating behavior / growth factor receptor binding / peptidase activator activity / Golgi-associated vesicle / PTB domain binding / positive regulation of amyloid fibril formation / Insertion of tail-anchored proteins into the endoplasmic reticulum membrane / astrocyte projection / Lysosome Vesicle Biogenesis / neuron remodeling / Deregulated CDK5 triggers multiple neurodegenerative pathways in Alzheimer's disease models / nuclear envelope lumen / dendrite development / positive regulation of protein metabolic process / TRAF6 mediated NF-kB activation / signaling receptor activator activity / negative regulation of long-term synaptic potentiation / Advanced glycosylation endproduct receptor signaling / modulation of excitatory postsynaptic potential / transition metal ion binding / main axon / The NLRP3 inflammasome / regulation of multicellular organism growth / intracellular copper ion homeostasis / ECM proteoglycans / regulation of presynapse assembly / positive regulation of T cell migration / neuronal dense core vesicle / response to insulin-like growth factor stimulus / Purinergic signaling in leishmaniasis infection / positive regulation of chemokine production / Notch signaling pathway / cellular response to manganese ion / swimming behavior / clathrin-coated pit / extracellular matrix organization / neuron projection maintenance / astrocyte activation / Mitochondrial protein degradation / ionotropic glutamate receptor signaling pathway / positive regulation of calcium-mediated signaling / positive regulation of mitotic cell cycle / protein serine/threonine kinase binding / axonogenesis / response to interleukin-1 / regulation of neuron apoptotic process / platelet alpha granule lumen / cellular response to copper ion / cellular response to cAMP / positive regulation of glycolytic process / central nervous system development / dendritic shaft / trans-Golgi network membrane / endosome lumen / positive regulation of long-term synaptic potentiation / positive regulation of interleukin-1 beta production / adult locomotory behavior / learning / Post-translational protein phosphorylation / locomotory behavior / serine-type endopeptidase inhibitor activity / microglial cell activation / positive regulation of non-canonical NF-kappaB signal transduction / cellular response to nerve growth factor stimulus / TAK1-dependent IKK and NF-kappa-B activation / recycling endosome / regulation of long-term neuronal synaptic plasticity / synapse organization / visual learning / positive regulation of JNK cascade / response to lead ion / Golgi lumen / positive regulation of interleukin-6 production / cognition / Regulation of Insulin-like Growth Factor (IGF) transport and uptake by Insulin-like Growth Factor Binding Proteins (IGFBPs) / cellular response to amyloid-beta / endocytosis / neuron projection development / positive regulation of tumor necrosis factor production / positive regulation of inflammatory response / calcium ion transport / Platelet degranulation / regulation of translation / heparin binding / regulation of gene expression Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method | helical reconstruction / cryo EM / Resolution: 3.2 Å | |||||||||
 Authors Authors | Wilkinson M / Leistner C / Burgess A / Goodfellow S / Deuchars S / Ranson NA / Radford SE / Frank RAW | |||||||||
| Funding support |  United Kingdom, 2 items United Kingdom, 2 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: The in-tissue molecular architecture of β-amyloid pathology in the mammalian brain. Authors: Conny Leistner / Martin Wilkinson / Ailidh Burgess / Megan Lovatt / Stanley Goodbody / Yong Xu / Susan Deuchars / Sheena E Radford / Neil A Ranson / René A W Frank /  Abstract: Amyloid plaques composed of Aβ fibrils are a hallmark of Alzheimer's disease (AD). However, the molecular architecture of amyloid plaques in the context of fresh mammalian brain tissue is unknown. ...Amyloid plaques composed of Aβ fibrils are a hallmark of Alzheimer's disease (AD). However, the molecular architecture of amyloid plaques in the context of fresh mammalian brain tissue is unknown. Here, using cryogenic correlated light and electron tomography we report the in situ molecular architecture of Aβ fibrils in the App familial AD mouse model containing the Arctic mutation and an atomic model of ex vivo purified Arctic Aβ fibrils. We show that in-tissue Aβ fibrils are arranged in a lattice or parallel bundles, and are interdigitated by subcellular compartments, extracellular vesicles, extracellular droplets and extracellular multilamellar bodies. The Arctic Aβ fibril differs significantly from an earlier App fibril structure, indicating a striking effect of the Arctic mutation. These structural data also revealed an ensemble of additional fibrillar species, including thin protofilament-like rods and branched fibrils. Together, these results provide a structural model for the dense network architecture that characterises β-amyloid plaque pathology. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_16019.map.gz emd_16019.map.gz | 5.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-16019-v30.xml emd-16019-v30.xml emd-16019.xml emd-16019.xml | 18.2 KB 18.2 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_16019.png emd_16019.png | 91.5 KB | ||
| Filedesc metadata |  emd-16019.cif.gz emd-16019.cif.gz | 5.8 KB | ||
| Others |  emd_16019_half_map_1.map.gz emd_16019_half_map_1.map.gz emd_16019_half_map_2.map.gz emd_16019_half_map_2.map.gz | 56.6 MB 56.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-16019 http://ftp.pdbj.org/pub/emdb/structures/EMD-16019 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-16019 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-16019 | HTTPS FTP |
-Related structure data
| Related structure data |  8bfbMC  8bfaC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_16019.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_16019.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | CryoEM map for extracted AppNLGF Abeta42 fibril after MOX04 labelling | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.94 Å | ||||||||||||||||||||||||||||||||||||
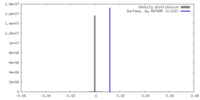
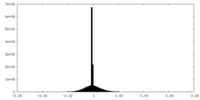
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: halfmap2
| File | emd_16019_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | halfmap2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
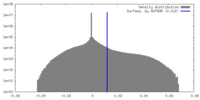
| Density Histograms |
-Half map: halfmap1
| File | emd_16019_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | halfmap1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
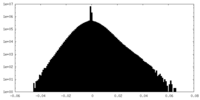
| Density Histograms |
- Sample components
Sample components
-Entire : Sarkosyl-extracted AppNL-G-F Abeta42 fibril
| Entire | Name: Sarkosyl-extracted AppNL-G-F Abeta42 fibril |
|---|---|
| Components |
|
-Supramolecule #1: Sarkosyl-extracted AppNL-G-F Abeta42 fibril
| Supramolecule | Name: Sarkosyl-extracted AppNL-G-F Abeta42 fibril / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all Details: Fibrils purified from mouse brain labelled with Methoxy-X04 |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 4.441 kDa/nm |
-Macromolecule #1: Amyloid-beta precursor protein
| Macromolecule | Name: Amyloid-beta precursor protein / type: protein_or_peptide / ID: 1 Details: Humanised Abeta42 from App^NL-G-F mice with arctic mutation (E22G) Number of copies: 10 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 4.448025 KDa |
| Sequence | String: DAEFRHDSGY EVHHQKLVFF AGDVGSNKGA IIGLMVGGVV IA UniProtKB: Amyloid-beta precursor protein |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | helical reconstruction |
| Aggregation state | filament |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 Component:
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Grid | Model: C-flat-1.2/1.3 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: PLASMA CLEANING / Pretreatment - Time: 60 sec. | |||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 90 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV / Details: 6s blot. | |||||||||
| Details | Sarkosyl-insoluble fibrils from App^NL-G-F mouse brain labelled with Methoxy-X04 |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: TFS Selectris / Energy filter - Slit width: 10 eV |
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Number grids imaged: 1 / Number real images: 4165 / Average exposure time: 6.0 sec. / Average electron dose: 41.0 e/Å2 Details: 1442 raw EER frames were collected per image and combined into 40 fractions for processing |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.9 µm / Nominal defocus min: 1.4000000000000001 µm / Nominal magnification: 130000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Final reconstruction | Applied symmetry - Helical parameters - Δz: 2.414 Å Applied symmetry - Helical parameters - Δ&Phi: 179.355 ° Applied symmetry - Helical parameters - Axial symmetry: C1 (asymmetric) Resolution.type: BY AUTHOR / Resolution: 3.2 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: RELION (ver. 4.0) / Number images used: 3640 |
|---|---|
| Segment selection | Number selected: 136214 / Software - Name: crYOLO Details: Manually picked a subset of images to train a model for automatic fibril segment picking in crYOLO |
| Startup model | Type of model: INSILICO MODEL Details: Model generated from 2D class averages using relion_helix_inimodel2d |
| Final angle assignment | Type: NOT APPLICABLE / Software - Name: RELION (ver. 4.0) |
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: AB INITIO MODEL / Overall B value: 52 / Target criteria: Correlation coefficient |
|---|---|
| Output model |  PDB-8bfb: |
 Movie
Movie Controller
Controller




















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)