[English] 日本語
 Yorodumi
Yorodumi- EMDB-15960: Ligand-Free Structure of the decameric sulfofructose transaldolas... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Ligand-Free Structure of the decameric sulfofructose transaldolase BmSF-TAL | ||||||||||||
 Map data Map data | |||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | transaldolase / cryo-EM / decamer / sulfofructose / TRANSFERASE | ||||||||||||
| Function / homology | 6-deoxy-6-sulfo-D-fructose transaldolase / Transaldolase/Fructose-6-phosphate aldolase, archaeal/bacterial / transaldolase activity / Transaldolase/Fructose-6-phosphate aldolase / Transaldolase/Fructose-6-phosphate aldolase / aldehyde-lyase activity / Aldolase-type TIM barrel / carbohydrate metabolic process / 6-deoxy-6-sulfo-D-fructose transaldolase Function and homology information Function and homology information | ||||||||||||
| Biological species |  Priestia megaterium DSM 319 (bacteria) / Priestia megaterium DSM 319 (bacteria) /  Bacillus aryabhattai (bacteria) Bacillus aryabhattai (bacteria) | ||||||||||||
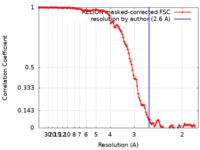
| Method | single particle reconstruction / cryo EM / Resolution: 2.6 Å | ||||||||||||
 Authors Authors | Snow AJD / Sharma M / Blaza J / Davies GJ | ||||||||||||
| Funding support |  United Kingdom, 3 items United Kingdom, 3 items
| ||||||||||||
 Citation Citation |  Journal: Structure / Year: 2023 Journal: Structure / Year: 2023Title: Structure and mechanism of sulfofructose transaldolase, a key enzyme in sulfoquinovose metabolism. Authors: Alexander J D Snow / Mahima Sharma / Palika Abayakoon / Spencer J Williams / James N Blaza / Gideon J Davies /   Abstract: Sulfoquinovose (SQ) is a key component of plant sulfolipids (sulfoquinovosyl diacylglycerols) and a major environmental reservoir of biological sulfur. Breakdown of SQ is achieved by bacteria through ...Sulfoquinovose (SQ) is a key component of plant sulfolipids (sulfoquinovosyl diacylglycerols) and a major environmental reservoir of biological sulfur. Breakdown of SQ is achieved by bacteria through the pathways of sulfoglycolysis. The sulfoglycolytic sulfofructose transaldolase (sulfo-SFT) pathway is used by gut-resident firmicutes and soil saprophytes. After isomerization of SQ to sulfofructose (SF), the namesake enzyme catalyzes the transaldol reaction of SF transferring dihydroxyacetone to 3C/4C acceptors to give sulfolactaldehyde and fructose-6-phosphate or sedoheptulose-7-phosphate. We report the 3D cryo-EM structure of SF transaldolase from Bacillus megaterium in apo and ligand bound forms, revealing a decameric structure formed from two pentameric rings of the protomer. We demonstrate a covalent "Schiff base" intermediate formed by reaction of SF with Lys89 within a conserved Asp-Lys-Glu catalytic triad and defined by an Arg-Trp-Arg sulfonate recognition triad. The structural characterization of the signature enzyme of the sulfo-SFT pathway provides key insights into molecular recognition of the sulfonate group of sulfosugars. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_15960.map.gz emd_15960.map.gz | 10.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-15960-v30.xml emd-15960-v30.xml emd-15960.xml emd-15960.xml | 21.5 KB 21.5 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_15960_fsc.xml emd_15960_fsc.xml | 11.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_15960.png emd_15960.png | 102.6 KB | ||
| Masks |  emd_15960_msk_1.map emd_15960_msk_1.map | 144.7 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-15960.cif.gz emd-15960.cif.gz | 7 KB | ||
| Others |  emd_15960_half_map_1.map.gz emd_15960_half_map_1.map.gz emd_15960_half_map_2.map.gz emd_15960_half_map_2.map.gz | 114.5 MB 114.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-15960 http://ftp.pdbj.org/pub/emdb/structures/EMD-15960 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-15960 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-15960 | HTTPS FTP |
-Validation report
| Summary document |  emd_15960_validation.pdf.gz emd_15960_validation.pdf.gz | 803.2 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_15960_full_validation.pdf.gz emd_15960_full_validation.pdf.gz | 802.7 KB | Display | |
| Data in XML |  emd_15960_validation.xml.gz emd_15960_validation.xml.gz | 19.2 KB | Display | |
| Data in CIF |  emd_15960_validation.cif.gz emd_15960_validation.cif.gz | 25.4 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15960 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15960 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15960 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15960 | HTTPS FTP |
-Related structure data
| Related structure data |  8bc2MC  8bc3C  8bc4C  8c4iC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_15960.map.gz / Format: CCP4 / Size: 144.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_15960.map.gz / Format: CCP4 / Size: 144.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.9 Å | ||||||||||||||||||||||||||||||||||||
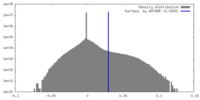
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_15960_msk_1.map emd_15960_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
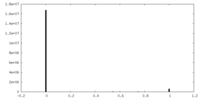
| Projections & Slices |
| ||||||||||||
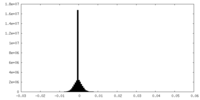
| Density Histograms |
-Half map: #2
| File | emd_15960_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
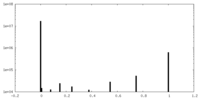
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_15960_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Decameric complex of BmSF-TAL in a ligand-free state.
| Entire | Name: Decameric complex of BmSF-TAL in a ligand-free state. |
|---|---|
| Components |
|
-Supramolecule #1: Decameric complex of BmSF-TAL in a ligand-free state.
| Supramolecule | Name: Decameric complex of BmSF-TAL in a ligand-free state. / type: cell / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Priestia megaterium DSM 319 (bacteria) Priestia megaterium DSM 319 (bacteria) |
-Macromolecule #1: Transaldolase
| Macromolecule | Name: Transaldolase / type: protein_or_peptide / ID: 1 / Number of copies: 10 / Enantiomer: LEVO / EC number: transaldolase |
|---|---|
| Source (natural) | Organism:  Bacillus aryabhattai (bacteria) Bacillus aryabhattai (bacteria) |
| Molecular weight | Theoretical: 24.443545 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MKYFLDSAIL EEIRYAYENW AIDGVTTNPR HIMNSGKPFL TVLDEFASEF KGVENFPISV EINPHLDNAK DMVEEGTKIA KLSSNFVIK IPCTEPGLIA AKEFEKQGIS TNVTLVFSPS QALQPARIGA KFVSPFVGWK ENSGDDTTYI QDIVNIYKNY N YNTEIIVA ...String: MKYFLDSAIL EEIRYAYENW AIDGVTTNPR HIMNSGKPFL TVLDEFASEF KGVENFPISV EINPHLDNAK DMVEEGTKIA KLSSNFVIK IPCTEPGLIA AKEFEKQGIS TNVTLVFSPS QALQPARIGA KFVSPFVGWK ENSGDDTTYI QDIVNIYKNY N YNTEIIVA ALRNGKQIVD AAKAGAHIVT CGFDVYKESF QHAFTDYGLN KFRNAWDNTV UniProtKB: 6-deoxy-6-sulfo-D-fructose transaldolase |
-Macromolecule #2: water
| Macromolecule | Name: water / type: ligand / ID: 2 / Number of copies: 460 / Formula: HOH |
|---|---|
| Molecular weight | Theoretical: 18.015 Da |
| Chemical component information |  ChemComp-HOH: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 2.5 mg/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.4 Component:
Details: Buffer was clarified in a 0.22um filter and used for final SEC polishing. | |||||||||
| Grid | Model: Quantifoil / Material: GOLD / Support film - Material: GOLD / Support film - topology: HOLEY | |||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | TFS GLACIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Digitization - Dimensions - Width: 4096 pixel / Digitization - Dimensions - Height: 4096 pixel / Number grids imaged: 1 / Number real images: 4690 / Average exposure time: 3.68 sec. / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 30.0 µm / Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.0 µm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
 Movie
Movie Controller
Controller





 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)