[English] 日本語
 Yorodumi
Yorodumi- EMDB-15856: Cryo-EM structure of the Neurospora crassa TOM core complex with ... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the Neurospora crassa TOM core complex with Tom20 over both pores | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Complex / outer membrane / mitochondria / membrane protein / TOM / translocase / neurospora crassa / TOM holo / Tom20 | |||||||||
| Biological species |  Neurospora crassa (fungus) Neurospora crassa (fungus) | |||||||||
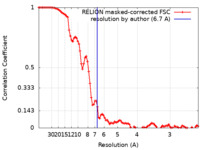
| Method | single particle reconstruction / cryo EM / Resolution: 6.7 Å | |||||||||
 Authors Authors | Ornelas P / Kuehlbrandt W | |||||||||
| Funding support |  Germany, 1 items Germany, 1 items
| |||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2023 Journal: Proc Natl Acad Sci U S A / Year: 2023Title: Two conformations of the Tom20 preprotein receptor in the TOM holo complex. Authors: Pamela Ornelas / Thomas Bausewein / Janosch Martin / Nina Morgner / Stephan Nussberger / Werner Kühlbrandt /  Abstract: The TOM complex is the main entry point for precursor proteins (preproteins) into mitochondria. Preproteins containing targeting sequences are recognized by the TOM complex and imported into ...The TOM complex is the main entry point for precursor proteins (preproteins) into mitochondria. Preproteins containing targeting sequences are recognized by the TOM complex and imported into mitochondria. We have determined the structure of the TOM core complex from by single-particle electron cryomicroscopy at 3.3 Å resolution, showing its interaction with a bound preprotein at 4 Å resolution, and of the TOM holo complex including the Tom20 receptor at 6 to 7 Å resolution. TOM is a transmembrane complex consisting of two β-barrels, three receptor subunits, and three short transmembrane subunits. Tom20 has a transmembrane helix and a receptor domain on the cytoplasmic side. We propose that Tom20 acts as a dynamic gatekeeper, guiding preproteins into the pores of the TOM complex. We analyze the interactions of Tom20 with other TOM subunits, present insights into the structure of the TOM holo complex, and suggest a translocation mechanism. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_15856.map.gz emd_15856.map.gz | 62.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-15856-v30.xml emd-15856-v30.xml emd-15856.xml emd-15856.xml | 12.7 KB 12.7 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_15856_fsc.xml emd_15856_fsc.xml | 9.3 KB | Display |  FSC data file FSC data file |
| Images |  emd_15856.png emd_15856.png | 141.2 KB | ||
| Others |  emd_15856_half_map_1.map.gz emd_15856_half_map_1.map.gz emd_15856_half_map_2.map.gz emd_15856_half_map_2.map.gz | 52.2 MB 52.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-15856 http://ftp.pdbj.org/pub/emdb/structures/EMD-15856 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-15856 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-15856 | HTTPS FTP |
-Validation report
| Summary document |  emd_15856_validation.pdf.gz emd_15856_validation.pdf.gz | 921.2 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_15856_full_validation.pdf.gz emd_15856_full_validation.pdf.gz | 920.7 KB | Display | |
| Data in XML |  emd_15856_validation.xml.gz emd_15856_validation.xml.gz | 16.2 KB | Display | |
| Data in CIF |  emd_15856_validation.cif.gz emd_15856_validation.cif.gz | 21.1 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15856 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15856 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15856 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15856 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_15856.map.gz / Format: CCP4 / Size: 67 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_15856.map.gz / Format: CCP4 / Size: 67 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.236 Å | ||||||||||||||||||||||||||||||||||||
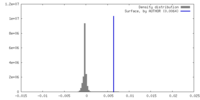
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_15856_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
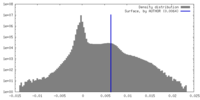
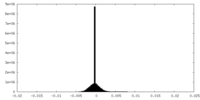
| Density Histograms |
-Half map: #2
| File | emd_15856_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
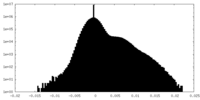
| Density Histograms |
- Sample components
Sample components
-Entire : Translocase of the outer mitochondrial membrane core complex of N...
| Entire | Name: Translocase of the outer mitochondrial membrane core complex of Neurospora Crassa |
|---|---|
| Components |
|
-Supramolecule #1: Translocase of the outer mitochondrial membrane core complex of N...
| Supramolecule | Name: Translocase of the outer mitochondrial membrane core complex of Neurospora Crassa type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#5 |
|---|---|
| Source (natural) | Organism:  Neurospora crassa (fungus) / Strain: GR-107 / Organelle: Mitochondria Neurospora crassa (fungus) / Strain: GR-107 / Organelle: Mitochondria |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Average electron dose: 55.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 3.0 µm / Nominal defocus min: 1.2 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller






 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)