[English] 日本語
 Yorodumi
Yorodumi- EMDB-15614: Structure of the SFTSV L protein stalled at late elongation [LATE... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of the SFTSV L protein stalled at late elongation [LATE-ELONGATION] | ||||||||||||
 Map data Map data | |||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | SFTSV RNA-DEPENDENT RNA POLYMERASE / VIRAL RNA / VIRAL PROTEIN | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationhost cell endoplasmic reticulum / virion component / host cell endoplasmic reticulum-Golgi intermediate compartment / host cell Golgi apparatus / RNA-directed RNA polymerase / viral RNA genome replication / RNA-directed RNA polymerase activity / DNA-templated transcription / metal ion binding Similarity search - Function | ||||||||||||
| Biological species |  SFTS virus AH12 SFTS virus AH12 | ||||||||||||
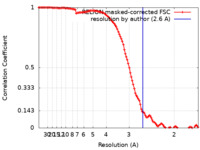
| Method | single particle reconstruction / cryo EM / Resolution: 2.6 Å | ||||||||||||
 Authors Authors | Williams HM / Thorkelsson SR / Vogel D / Milewski M / Busch C / Cusack S / Grunewald K / Quemin ERJ / Rosenthal M | ||||||||||||
| Funding support |  Germany, 3 items Germany, 3 items
| ||||||||||||
 Citation Citation |  Journal: Nucleic Acids Res / Year: 2023 Journal: Nucleic Acids Res / Year: 2023Title: Structural insights into viral genome replication by the severe fever with thrombocytopenia syndrome virus L protein. Authors: Harry M Williams / Sigurdur R Thorkelsson / Dominik Vogel / Morlin Milewski / Carola Busch / Stephen Cusack / Kay Grünewald / Emmanuelle R J Quemin / Maria Rosenthal /   Abstract: Severe fever with thrombocytopenia syndrome virus (SFTSV) is a phenuivirus that has rapidly become endemic in several East Asian countries. The large (L) protein of SFTSV, which includes the RNA- ...Severe fever with thrombocytopenia syndrome virus (SFTSV) is a phenuivirus that has rapidly become endemic in several East Asian countries. The large (L) protein of SFTSV, which includes the RNA-dependent RNA polymerase (RdRp), is responsible for catalysing viral genome replication and transcription. Here, we present 5 cryo-electron microscopy (cryo-EM) structures of the L protein in several states of the genome replication process, from pre-initiation to late-stage elongation, at a resolution of up to 2.6 Å. We identify how the L protein binds the 5' viral RNA in a hook-like conformation and show how the distal 5' and 3' RNA ends form a duplex positioning the 3' RNA terminus in the RdRp active site ready for initiation. We also observe the L protein stalled in the early and late stages of elongation with the RdRp core accommodating a 10-bp product-template duplex. This duplex ultimately splits with the template binding to a designated 3' secondary binding site. The structural data and observations are complemented by in vitro biochemical and cell-based mini-replicon assays. Altogether, our data provide novel key insights into the mechanism of viral genome replication by the SFTSV L protein and will aid drug development against segmented negative-strand RNA viruses. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_15614.map.gz emd_15614.map.gz | 117.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-15614-v30.xml emd-15614-v30.xml emd-15614.xml emd-15614.xml | 25.9 KB 25.9 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_15614_fsc.xml emd_15614_fsc.xml | 11.3 KB | Display |  FSC data file FSC data file |
| Images |  emd_15614.png emd_15614.png | 93.6 KB | ||
| Filedesc metadata |  emd-15614.cif.gz emd-15614.cif.gz | 8.7 KB | ||
| Others |  emd_15614_half_map_1.map.gz emd_15614_half_map_1.map.gz emd_15614_half_map_2.map.gz emd_15614_half_map_2.map.gz | 98.5 MB 98.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-15614 http://ftp.pdbj.org/pub/emdb/structures/EMD-15614 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-15614 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-15614 | HTTPS FTP |
-Validation report
| Summary document |  emd_15614_validation.pdf.gz emd_15614_validation.pdf.gz | 1.2 MB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_15614_full_validation.pdf.gz emd_15614_full_validation.pdf.gz | 1.2 MB | Display | |
| Data in XML |  emd_15614_validation.xml.gz emd_15614_validation.xml.gz | 18.4 KB | Display | |
| Data in CIF |  emd_15614_validation.cif.gz emd_15614_validation.cif.gz | 24.5 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15614 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15614 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15614 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15614 | HTTPS FTP |
-Related structure data
| Related structure data |  8asdMC  8as6C  8as7C  8asbC  8asgC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_15614.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_15614.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.85 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_15614_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_15614_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
+Entire : Structure of the SFTSV L protein stalled at late elongation [LATE...
+Supramolecule #1: Structure of the SFTSV L protein stalled at late elongation [LATE...
+Supramolecule #2: RNA-dependent RNA-polymerase L protein
+Supramolecule #3: RNA
+Macromolecule #1: RNA-directed RNA polymerase
+Macromolecule #2: RNA (5'-R(P*AP*AP*AP*AP*AP*AP*GP*AP*UP*CP*UP*GP*GP*GP*CP*GP*GP*UP...
+Macromolecule #3: RNA (5'-R(P*AP*CP*AP*CP*AP*AP*AP*GP*AP*CP*CP*GP*CP*CP*CP*AP*GP*AP...
+Macromolecule #4: RNA (5'-R(P*AP*CP*AP*CP*AP*GP*AP*GP*AP*CP*GP*CP*CP*CP*AP*GP*AP*UP...
+Macromolecule #5: MAGNESIUM ION
+Macromolecule #6: 5'-O-[(S)-hydroxy{[(S)-hydroxy(phosphonooxy)phosphoryl]amino}phos...
+Macromolecule #7: water
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7 |
|---|---|
| Vitrification | Cryogen name: ETHANE-PROPANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 53.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.5 µm / Nominal defocus min: 0.8 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller







 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)































 Trichoplusia ni (cabbage looper)
Trichoplusia ni (cabbage looper)