+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Bunyamwera Virus Gn/Gc Glycoprotein pH 6.3/K+ Treated | ||||||||||||||||||
 Map data Map data | |||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||
 Keywords Keywords | Glycoprotein / fusion protein / orthobunyavirus / primed / VIRAL PROTEIN | ||||||||||||||||||
| Biological species |  Bunyamwera virus Bunyamwera virus | ||||||||||||||||||
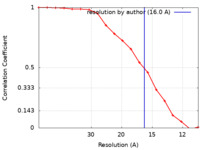
| Method | subtomogram averaging / cryo EM / Resolution: 16.0 Å | ||||||||||||||||||
 Authors Authors | Hover S / Fontana J | ||||||||||||||||||
| Funding support |  United Kingdom, United Kingdom,  France, 5 items France, 5 items
| ||||||||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: Organisation of the orthobunyavirus tripodal spike and the structural changes induced by low pH and K during entry. Authors: Samantha Hover / Frank W Charlton / Jan Hellert / Jessica J Swanson / Jamel Mankouri / John N Barr / Juan Fontana /   Abstract: Following endocytosis, enveloped viruses employ the changing environment of maturing endosomes as cues to promote endosomal escape, a process often mediated by viral glycoproteins. We previously ...Following endocytosis, enveloped viruses employ the changing environment of maturing endosomes as cues to promote endosomal escape, a process often mediated by viral glycoproteins. We previously showed that both high [K] and low pH promote entry of Bunyamwera virus (BUNV), the prototypical bunyavirus. Here, we use sub-tomogram averaging and AlphaFold, to generate a pseudo-atomic model of the whole BUNV glycoprotein envelope. We unambiguously locate the Gc fusion domain and its chaperone Gn within the floor domain of the spike. Furthermore, viral incubation at low pH and high [K], reminiscent of endocytic conditions, results in a dramatic rearrangement of the BUNV envelope. Structural and biochemical assays indicate that pH 6.3/K in the absence of a target membrane elicits a fusion-capable triggered intermediate state of BUNV GPs; but the same conditions induce fusion when target membranes are present. Taken together, we provide mechanistic understanding of the requirements for bunyavirus entry. | ||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_15579.map.gz emd_15579.map.gz | 241.1 KB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-15579-v30.xml emd-15579-v30.xml emd-15579.xml emd-15579.xml | 14.5 KB 14.5 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_15579_fsc.xml emd_15579_fsc.xml | 1.6 KB | Display |  FSC data file FSC data file |
| Images |  emd_15579.png emd_15579.png | 50.8 KB | ||
| Filedesc metadata |  emd-15579.cif.gz emd-15579.cif.gz | 4.1 KB | ||
| Others |  emd_15579_half_map_1.map.gz emd_15579_half_map_1.map.gz emd_15579_half_map_2.map.gz emd_15579_half_map_2.map.gz | 242.8 KB 242.9 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-15579 http://ftp.pdbj.org/pub/emdb/structures/EMD-15579 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-15579 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-15579 | HTTPS FTP |
-Validation report
| Summary document |  emd_15579_validation.pdf.gz emd_15579_validation.pdf.gz | 638.9 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_15579_full_validation.pdf.gz emd_15579_full_validation.pdf.gz | 638.4 KB | Display | |
| Data in XML |  emd_15579_validation.xml.gz emd_15579_validation.xml.gz | 6.6 KB | Display | |
| Data in CIF |  emd_15579_validation.cif.gz emd_15579_validation.cif.gz | 8.5 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15579 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15579 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15579 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15579 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_15579.map.gz / Format: CCP4 / Size: 262.7 KB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_15579.map.gz / Format: CCP4 / Size: 262.7 KB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. generated in cubic-lattice coordinate | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 5.44 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_15579_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_15579_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Bunyamwera virus
| Entire | Name:  Bunyamwera virus Bunyamwera virus |
|---|---|
| Components |
|
-Supramolecule #1: Bunyamwera virus
| Supramolecule | Name: Bunyamwera virus / type: virus / ID: 1 / Parent: 0 / NCBI-ID: 35304 / Sci species name: Bunyamwera virus / Virus type: VIRION / Virus isolate: SPECIES / Virus enveloped: Yes / Virus empty: No |
|---|
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | subtomogram averaging |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 6.3 Component:
Details: Virions were treated for 2 hrs in pH 6.3/K+ buffer, prior to vitrification | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vitrification | Cryogen name: ETHANE | ||||||||||||
| Details | Virions pH 6.3/K+ treated prior to vitrification |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 1.8 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.0 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller






 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)