[English] 日本語
 Yorodumi
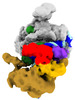
Yorodumi- EMDB-15006: Structure of SNAPc containing Pol II pre-initiation complex bound... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of SNAPc containing Pol II pre-initiation complex bound to U1 snRNA promoter (CC) | |||||||||||||||
 Map data Map data | ||||||||||||||||
 Sample Sample |
| |||||||||||||||
 Keywords Keywords | RNA polymerase II / Pol II / PIC / SNAPc / snRNA / U1 promoter / TRANSCRIPTION | |||||||||||||||
| Function / homology |  Function and homology information Function and homology informationsnRNA-activating protein complex / snRNA transcription / snRNA transcription by RNA polymerase III / bent DNA binding / : / positive regulation of core promoter binding / RNA polymerase II core complex assembly / RNA polymerase transcription factor SL1 complex / RNA polymerase III type 3 promoter sequence-specific DNA binding / meiotic sister chromatid cohesion ...snRNA-activating protein complex / snRNA transcription / snRNA transcription by RNA polymerase III / bent DNA binding / : / positive regulation of core promoter binding / RNA polymerase II core complex assembly / RNA polymerase transcription factor SL1 complex / RNA polymerase III type 3 promoter sequence-specific DNA binding / meiotic sister chromatid cohesion / phosphatase activator activity / snRNA transcription by RNA polymerase II / RNA polymerase III general transcription initiation factor activity / TFIIF-class transcription factor complex binding / transcriptional start site selection at RNA polymerase II promoter / RNA polymerase I core promoter sequence-specific DNA binding / transcription factor TFIIF complex / RNA Polymerase III Transcription Initiation From Type 1 Promoter / RNA Polymerase III Transcription Initiation From Type 2 Promoter / RNA Polymerase III Transcription Initiation From Type 3 Promoter / Formation of RNA Pol II elongation complex / Formation of the Early Elongation Complex / Transcriptional regulation by small RNAs / RNA Polymerase II Pre-transcription Events / TP53 Regulates Transcription of DNA Repair Genes / FGFR2 alternative splicing / RNA polymerase II transcribes snRNA genes / mRNA Capping / mRNA Splicing - Minor Pathway / Processing of Capped Intron-Containing Pre-mRNA / RNA Polymerase II Promoter Escape / RNA Polymerase II Transcription Pre-Initiation And Promoter Opening / RNA Polymerase II Transcription Initiation / RNA Polymerase II Transcription Elongation / RNA Polymerase II Transcription Initiation And Promoter Clearance / RNA Pol II CTD phosphorylation and interaction with CE / Estrogen-dependent gene expression / Formation of TC-NER Pre-Incision Complex / Dual incision in TC-NER / Gap-filling DNA repair synthesis and ligation in TC-NER / mRNA Splicing - Major Pathway / female germ cell nucleus / transcription factor TFIIA complex / RNA Polymerase III Abortive And Retractive Initiation / male pronucleus / female pronucleus / germinal vesicle / RNA polymerase II general transcription initiation factor binding / nuclear thyroid hormone receptor binding / Abortive elongation of HIV-1 transcript in the absence of Tat / FGFR2 alternative splicing / transcription preinitiation complex / RNA Polymerase I Transcription Termination / Viral Messenger RNA Synthesis / Signaling by FGFR2 IIIa TM / protein acetylation / RNA polymerase II general transcription initiation factor activity / transcription factor TFIID complex / cell division site / acetyltransferase activity / positive regulation of nuclear-transcribed mRNA poly(A) tail shortening / RNA Pol II CTD phosphorylation and interaction with CE during HIV infection / RNA Pol II CTD phosphorylation and interaction with CE / Formation of the Early Elongation Complex / Formation of the HIV-1 Early Elongation Complex / mRNA Capping / transcription initiation at RNA polymerase III promoter / organelle membrane / HIV Transcription Initiation / RNA Polymerase II HIV Promoter Escape / Transcription of the HIV genome / RNA Polymerase II Promoter Escape / RNA Polymerase II Transcription Pre-Initiation And Promoter Opening / RNA Polymerase II Transcription Initiation / RNA Polymerase II Transcription Initiation And Promoter Clearance / mRNA Splicing - Minor Pathway / aryl hydrocarbon receptor binding / histone acetyltransferase activity / viral transcription / TFIIB-class transcription factor binding / RNA polymerase II complex binding / maintenance of transcriptional fidelity during transcription elongation by RNA polymerase II / RNA Polymerase I Transcription Initiation / Processing of Capped Intron-Containing Pre-mRNA / transcription by RNA polymerase III / positive regulation of translational initiation / Pausing and recovery of Tat-mediated HIV elongation / Tat-mediated HIV elongation arrest and recovery / RNA polymerase II transcribes snRNA genes / HIV elongation arrest and recovery / Pausing and recovery of HIV elongation / positive regulation of transcription initiation by RNA polymerase II / negative regulation of protein binding / Tat-mediated elongation of the HIV-1 transcript / RNA polymerase I complex / RNA polymerase III complex / Formation of HIV-1 elongation complex containing HIV-1 Tat / core promoter sequence-specific DNA binding / transcription elongation by RNA polymerase I / RNA polymerase II core promoter sequence-specific DNA binding Similarity search - Function | |||||||||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /  | |||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.4 Å | |||||||||||||||
 Authors Authors | Rengachari S / Schilbach S / Kaliyappan T / Gouge J / Zumer K / Schwarz J / Urlaub H / Dienemann C / Vannini A / Cramer P | |||||||||||||||
| Funding support |  Germany, Germany,  United Kingdom, 4 items United Kingdom, 4 items
| |||||||||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2022 Journal: Nat Struct Mol Biol / Year: 2022Title: Structural basis of SNAPc-dependent snRNA transcription initiation by RNA polymerase II. Authors: Srinivasan Rengachari / Sandra Schilbach / Thangavelu Kaliyappan / Jerome Gouge / Kristina Zumer / Juliane Schwarz / Henning Urlaub / Christian Dienemann / Alessandro Vannini / Patrick Cramer /    Abstract: RNA polymerase II (Pol II) carries out transcription of both protein-coding and non-coding genes. Whereas Pol II initiation at protein-coding genes has been studied in detail, Pol II initiation at ...RNA polymerase II (Pol II) carries out transcription of both protein-coding and non-coding genes. Whereas Pol II initiation at protein-coding genes has been studied in detail, Pol II initiation at non-coding genes, such as small nuclear RNA (snRNA) genes, is less well understood at the structural level. Here, we study Pol II initiation at snRNA gene promoters and show that the snRNA-activating protein complex (SNAPc) enables DNA opening and transcription initiation independent of TFIIE and TFIIH in vitro. We then resolve cryo-EM structures of the SNAPc-containing Pol IIpre-initiation complex (PIC) assembled on U1 and U5 snRNA promoters. The core of SNAPc binds two turns of DNA and recognizes the snRNA promoter-specific proximal sequence element (PSE), located upstream of the TATA box-binding protein TBP. Two extensions of SNAPc, called wing-1 and wing-2, bind TFIIA and TFIIB, respectively, explaining how SNAPc directs Pol II to snRNA promoters. Comparison of structures of closed and open promoter complexes elucidates TFIIH-independent DNA opening. These results provide the structural basis of Pol II initiation at non-coding RNA gene promoters. #1:  Journal: Biorxiv / Year: 2022 Journal: Biorxiv / Year: 2022Title: Structural basis of SNAPc-dependent snRNA transcription initiation by RNA polymerase II Authors: Rengachari S / Schilbach S / Kaliyappan T / Gouge J / Zumer K / Schwarz J / Urlaub H / Dienemann C / Vannini A / Cramer P | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_15006.map.gz emd_15006.map.gz | 141.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-15006-v30.xml emd-15006-v30.xml emd-15006.xml emd-15006.xml | 51.3 KB 51.3 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_15006_fsc.xml emd_15006_fsc.xml | 14.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_15006.png emd_15006.png | 94.1 KB | ||
| Masks |  emd_15006_msk_1.map emd_15006_msk_1.map | 244.1 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-15006.cif.gz emd-15006.cif.gz | 12.9 KB | ||
| Others |  emd_15006_half_map_1.map.gz emd_15006_half_map_1.map.gz emd_15006_half_map_2.map.gz emd_15006_half_map_2.map.gz | 193.9 MB 194.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-15006 http://ftp.pdbj.org/pub/emdb/structures/EMD-15006 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-15006 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-15006 | HTTPS FTP |
-Related structure data
| Related structure data |  7zx7MC  7zwcC  7zwdC  7zx8C  7zxeC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_15006.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_15006.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.05 Å | ||||||||||||||||||||||||||||||||||||
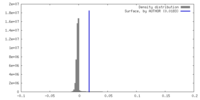
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_15006_msk_1.map emd_15006_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
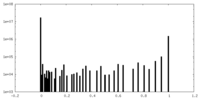
| Projections & Slices |
| ||||||||||||
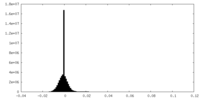
| Density Histograms |
-Half map: #2
| File | emd_15006_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_15006_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
+Entire : Pre-initiation complex of RNA polymerase II with general transcri...
+Supramolecule #1: Pre-initiation complex of RNA polymerase II with general transcri...
+Supramolecule #2: SNAPc
+Supramolecule #3: TATA-box-binding protein and transcription factors
+Supramolecule #4: Promoter
+Supramolecule #5: RNA polymerase II
+Macromolecule #1: DNA-directed RNA polymerase subunit
+Macromolecule #2: DNA-directed RNA polymerase subunit beta
+Macromolecule #3: DNA-directed RNA polymerase II subunit RPB3
+Macromolecule #4: RNA polymerase II subunit D
+Macromolecule #5: DNA-directed RNA polymerase II subunit E
+Macromolecule #6: DNA-directed RNA polymerase II subunit F
+Macromolecule #7: DNA-directed RNA polymerase II subunit RPB7
+Macromolecule #8: DNA-directed RNA polymerases I, II, and III subunit RPABC3
+Macromolecule #9: DNA-directed RNA polymerase II subunit RPB9
+Macromolecule #10: DNA-directed RNA polymerases I, II, and III subunit RPABC5
+Macromolecule #11: RNA polymerase II subunit J
+Macromolecule #12: RNA polymerase II subunit K
+Macromolecule #13: Transcription initiation factor IIB
+Macromolecule #15: TATA-box-binding protein
+Macromolecule #16: General transcription factor IIF subunit 1
+Macromolecule #17: General transcription factor IIF subunit 2
+Macromolecule #19: Transcription initiation factor IIA subunit 1
+Macromolecule #20: Transcription initiation factor IIA subunit 2
+Macromolecule #21: snRNA-activating protein complex subunit 1
+Macromolecule #22: snRNA-activating protein complex subunit 3
+Macromolecule #23: snRNA-activating protein complex subunit 4
+Macromolecule #24: snRNA-activating protein complex subunit 5
+Macromolecule #14: Non-template strand
+Macromolecule #18: Template strand
+Macromolecule #25: ZINC ION
+Macromolecule #26: MAGNESIUM ION
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 54.45 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.5 µm / Nominal defocus min: 0.3 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller



























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)









































 Trichoplusia ni (cabbage looper)
Trichoplusia ni (cabbage looper)
