[English] 日本語
 Yorodumi
Yorodumi- EMDB-1434: Nautilus pompilius hemocyanin: 9 A cryo-EM structure and molecula... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-1434 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Nautilus pompilius hemocyanin: 9 A cryo-EM structure and molecular model reveal the subunit pathway and the interfaces between the 70 functional units. | |||||||||
 Map data Map data | This is a map of the hemocyanin from the mollusc Nautilus pompilius. Mass correlated threshold: 0.006 | |||||||||
 Sample Sample |
| |||||||||
| Biological species |  Nautilus pompilius (invertebrata) Nautilus pompilius (invertebrata) | |||||||||
| Method | single particle reconstruction / cryo EM / negative staining / Resolution: 9.1 Å | |||||||||
 Authors Authors | Gatsogiannis C / Moeller A / Depoix F / Meissner U / Markl J | |||||||||
 Citation Citation |  Journal: J Mol Biol / Year: 2007 Journal: J Mol Biol / Year: 2007Title: Nautilus pompilius hemocyanin: 9 A cryo-EM structure and molecular model reveal the subunit pathway and the interfaces between the 70 functional units. Authors: Christos Gatsogiannis / Arne Moeller / Frank Depoix / Ulrich Meissner / Jürgen Markl /  Abstract: Hemocyanins are giant extracellular oxygen carriers in the hemolymph of many molluscs. Nautilus pompilius (Cephalopoda) hemocyanin is a cylindrical decamer of a 350 kDa polypeptide subunit that in ...Hemocyanins are giant extracellular oxygen carriers in the hemolymph of many molluscs. Nautilus pompilius (Cephalopoda) hemocyanin is a cylindrical decamer of a 350 kDa polypeptide subunit that in turn is a "pearl-chain" of seven different functional units (FU-a to FU-g). Each globular FU has a binuclear copper centre that reversibly binds one O(2) molecule, and the 70-FU decamer is a highly allosteric protein. Its primary structure and an 11 A cryo-electron microscopy (cryo-EM) structure have recently been determined, and the crystal structures of two related FU types are available in the databanks. However, in molluscan hemocyanin, the precise subunit pathway within the decamer, the inter-FU interfaces, and the allosteric unit are still obscure, but this knowledge is crucial to understand assembly and allosterism of these proteins. Here we present the cryo-EM structure of Nautilus hemocyanin at 9.1 A resolution (FSC(1/2-bit) criterion), and its molecular model obtained by rigid-body fitting of the individual FUs. In this model we identified the subunit dimer, the subunit pathway, and 15 types of inter-FU interface. Four interface types correspond to the association mode of the two protomers in the published Octopus FU-g crystal. Other interfaces explain previously described morphological structures such as the fenestrated wall (which shows D5 symmetry), the three horizontal wall tiers, the major and minor grooves, the anchor structure and the internal collar (which unexpectedly has C5 symmetry). Moreover, the potential calcium/magnesium and N-glycan binding sites have emerged. Many interfaces have amino acid constellations that might transfer allosteric interaction between FUs. From their topologies we propose that the prime allosteric unit is the oblique segment between major and minor groove, consisting of seven FUs from two different subunits. Thus, the 9 A structure of Nautilus hemocyanin provides fundamentally new insight into the architecture and function of molluscan hemocyanins. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_1434.map.gz emd_1434.map.gz | 60.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-1434-v30.xml emd-1434-v30.xml emd-1434.xml emd-1434.xml | 9.2 KB 9.2 KB | Display Display |  EMDB header EMDB header |
| Images |  em-1434.png em-1434.png | 422 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-1434 http://ftp.pdbj.org/pub/emdb/structures/EMD-1434 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-1434 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-1434 | HTTPS FTP |
-Validation report
| Summary document |  emd_1434_validation.pdf.gz emd_1434_validation.pdf.gz | 234.5 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_1434_full_validation.pdf.gz emd_1434_full_validation.pdf.gz | 233.6 KB | Display | |
| Data in XML |  emd_1434_validation.xml.gz emd_1434_validation.xml.gz | 6.3 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-1434 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-1434 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-1434 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-1434 | HTTPS FTP |
-Related structure data
| Similar structure data |
|---|
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_1434.map.gz / Format: CCP4 / Size: 62.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_1434.map.gz / Format: CCP4 / Size: 62.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | This is a map of the hemocyanin from the mollusc Nautilus pompilius. Mass correlated threshold: 0.006 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.85 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
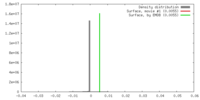
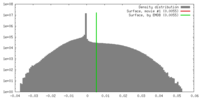
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Nautilus pompilius hemocyanin
| Entire | Name: Nautilus pompilius hemocyanin |
|---|---|
| Components |
|
-Supramolecule #1000: Nautilus pompilius hemocyanin
| Supramolecule | Name: Nautilus pompilius hemocyanin / type: sample / ID: 1000 Oligomeric state: Nautilus pompilius hemocyanin is a decamer of a 350 kDa subunit. Each subunit is composed by 7 paralogous O2 binding functional units. Number unique components: 1 |
|---|---|
| Molecular weight | Theoretical: 3.5 MDa |
-Macromolecule #1: Nautilus pompilius hemocyanin
| Macromolecule | Name: Nautilus pompilius hemocyanin / type: protein_or_peptide / ID: 1 / Name.synonym: Nautilus pompilius hemocyanin / Recombinant expression: No |
|---|---|
| Source (natural) | Organism:  Nautilus pompilius (invertebrata) / Tissue: hemolymph Nautilus pompilius (invertebrata) / Tissue: hemolymph |
| Molecular weight | Experimental: 3.5 MDa |
-Experimental details
-Structure determination
| Method | negative staining, cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.4 mg/mL |
|---|---|
| Buffer | pH: 7.4 / Details: 50mM Tris-HCl, 5mM CaCl2, 5mM MgCl2, 150mM NaCl |
| Staining | Type: NEGATIVE / Details: cryo-EM, no stain |
| Grid | Details: 400 mesh copper |
| Vitrification | Cryogen name: ETHANE / Chamber temperature: 86 K / Instrument: HOMEMADE PLUNGER Details: Vitrification instrument: home made. Vitrification carried out in 100 percent nitrogen atmosphere Method: Single side blotting and rapid plunging |
- Electron microscopy
Electron microscopy
| Microscope | FEI TECNAI F30 |
|---|---|
| Temperature | Average: 86 K |
| Image recording | Category: FILM / Film or detector model: KODAK SO-163 FILM / Digitization - Scanner: PRIMESCAN / Digitization - Sampling interval: 1.86 µm / Number real images: 63 / Bits/pixel: 8 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated magnification: 49000 / Illumination mode: OTHER / Imaging mode: BRIGHT FIELD / Cs: 1.2 mm / Nominal defocus max: 3.3 µm / Nominal defocus min: 0.8 µm / Nominal magnification: 49000 |
| Sample stage | Specimen holder: Gatan single-tilt cryoholder / Specimen holder model: GATAN LIQUID NITROGEN |
| Experimental equipment |  Model: Tecnai F30 / Image courtesy: FEI Company |
- Image processing
Image processing
| Details | The particles were selected using the automatic selection program boxer |
|---|---|
| CTF correction | Details: CTFFIND3 and TRANSFER, IMAGIC 5 |
| Final reconstruction | Applied symmetry - Point group: C5 (5 fold cyclic) / Algorithm: OTHER / Resolution.type: BY AUTHOR / Resolution: 9.1 Å / Resolution method: FSC 0.5 CUT-OFF / Software - Name: IMAGIC-5 / Number images used: 16000 |
| Final two d classification | Number classes: 5200 |
 Movie
Movie Controller
Controller







 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)