[English] 日本語
 Yorodumi
Yorodumi- EMDB-1403: Cryo-electron microscopy of hepatitis B virions reveals variabili... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-1403 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-electron microscopy of hepatitis B virions reveals variability in envelope capsid interactions. | |||||||||
 Map data Map data | 3d MAP OF SUB-SET OF HEPATITIS B VIRUS Particles (COMPACT PARTICLES; grouped in the same class) | |||||||||
 Sample Sample |
| |||||||||
| Biological species |   Hepatitis B virus Hepatitis B virus | |||||||||
| Method | single particle reconstruction / cryo EM | |||||||||
 Authors Authors | Seitz S / Urban S / Antoni C / Bottcher B | |||||||||
 Citation Citation |  Journal: EMBO J / Year: 2007 Journal: EMBO J / Year: 2007Title: Cryo-electron microscopy of hepatitis B virions reveals variability in envelope capsid interactions. Authors: Stefan Seitz / Stephan Urban / Christoph Antoni / Bettina Böttcher /  Abstract: Hepatitis B virus (HBV) is a major human pathogen causing about 750,000 deaths per year. The virion consists of a nucleocapsid and an envelope formed by lipids, and three integral membrane proteins. ...Hepatitis B virus (HBV) is a major human pathogen causing about 750,000 deaths per year. The virion consists of a nucleocapsid and an envelope formed by lipids, and three integral membrane proteins. Although we have detailed structural insights into the organization of the HBV core, the arrangement of the envelope in virions and its interaction with the nucleocapsid is elusive. Here we show the ultrastructure of hepatitis B virions purified from patient serum. We identified two morphological phenotypes, which appear as compact and gapped particles with nucleocapsids in distinguishable conformations. The overall structures of these nucleocapsids resemble recombinant cores with two alpha-helical spikes per asymmetric unit. At the charged tips the spikes are contacted by defined protrusions of the envelope proteins, probably via electrostatic interactions. The HBV envelope in the two morphotypes is to some extent variable, but the surface proteins follow a general packing scheme with up to three surface protein dimers per asymmetric unit. The variability in the structure of the envelope indicates that the nucleocapsid does not firmly constrain the arrangement of the surface proteins, but provides a general template for the packing. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_1403.map.gz emd_1403.map.gz | 527.4 KB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-1403-v30.xml emd-1403-v30.xml emd-1403.xml emd-1403.xml | 8.4 KB 8.4 KB | Display Display |  EMDB header EMDB header |
| Images |  1403.gif 1403.gif | 11.1 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-1403 http://ftp.pdbj.org/pub/emdb/structures/EMD-1403 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-1403 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-1403 | HTTPS FTP |
-Related structure data
| Related structure data |  1399C  1400C  1401C  1402C  1404C  1405C  1406C  1407C  1408C C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_1403.map.gz / Format: CCP4 / Size: 955.1 KB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_1403.map.gz / Format: CCP4 / Size: 955.1 KB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | 3d MAP OF SUB-SET OF HEPATITIS B VIRUS Particles (COMPACT PARTICLES; grouped in the same class) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 8.4 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
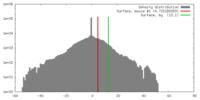
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Hepatitis B virus
| Entire | Name:   Hepatitis B virus Hepatitis B virus |
|---|---|
| Components |
|
-Supramolecule #1000: Hepatitis B virus
| Supramolecule | Name: Hepatitis B virus / type: sample / ID: 1000 / Details: virus purified from patients serum / Number unique components: 1 |
|---|
-Supramolecule #1: Hepatitis B virus
| Supramolecule | Name: Hepatitis B virus / type: virus / ID: 1 / Name.synonym: HBV / NCBI-ID: 10407 / Sci species name: Hepatitis B virus / Virus type: VIRION / Virus isolate: SPECIES / Virus enveloped: Yes / Virus empty: No / Syn species name: HBV |
|---|---|
| Host (natural) | Organism:  Homo sapiens (human) / synonym: VERTEBRATES Homo sapiens (human) / synonym: VERTEBRATES |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Grid | Details: 400 mesh copper rhodium grids (Maxtaform), |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 90 % / Chamber temperature: 93 K / Instrument: HOMEMADE PLUNGER / Details: Vitrification instrument: self made Method: automatic blot for 1-2 seconds from both sides before plunging |
- Electron microscopy
Electron microscopy
| Microscope | FEI/PHILIPS CM120T |
|---|---|
| Temperature | Average: 178 K |
| Alignment procedure | Legacy - Astigmatism: corrected at 200,000 times magnification |
| Image recording | Category: FILM / Film or detector model: KODAK SO-163 FILM / Digitization - Scanner: ZEISS SCAI / Digitization - Sampling interval: 21 µm / Number real images: 74 / Average electron dose: 15 e/Å2 / Bits/pixel: 8 |
| Electron beam | Acceleration voltage: 100 kV / Electron source: LAB6 |
| Electron optics | Calibrated magnification: 50000 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 6.3 mm / Nominal defocus max: 2.6 µm / Nominal defocus min: 0.9 µm / Nominal magnification: 52000 |
| Sample stage | Specimen holder: Side entry liquid nitrogen-cooled cryo specimen holder Specimen holder model: GATAN LIQUID NITROGEN |
- Image processing
Image processing
| Details | particles were selected manually |
|---|---|
| CTF correction | Details: Each Particle |
| Final reconstruction | Applied symmetry - Point group: I (icosahedral) / Algorithm: OTHER / Software - Name: MRC / Number images used: 16 |
 Movie
Movie Controller
Controller


 UCSF Chimera
UCSF Chimera

 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)