[English] 日本語
 Yorodumi
Yorodumi- EMDB-13308: Cryo-EM structure of the GroEL-GroES complex with ADP bound to bo... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-13308 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the GroEL-GroES complex with ADP bound to both rings ("tight" conformation). | |||||||||
 Map data Map data | Locally sharpened C7 map of "tight" conformation of the GroEL-GroES complex. ADP bound to both rings. | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | cryo-EM / chaperonin / GroEL / GroEL-GroES / CHAPERONE | |||||||||
| Function / homology |  Function and homology information Function and homology informationGroEL-GroES complex / chaperonin ATPase / virion assembly / : / isomerase activity / protein folding chaperone / ATP-dependent protein folding chaperone / response to radiation / unfolded protein binding / protein-folding chaperone binding ...GroEL-GroES complex / chaperonin ATPase / virion assembly / : / isomerase activity / protein folding chaperone / ATP-dependent protein folding chaperone / response to radiation / unfolded protein binding / protein-folding chaperone binding / protein folding / response to heat / protein refolding / magnesium ion binding / ATP hydrolysis activity / ATP binding / metal ion binding / identical protein binding / membrane / cytosol Similarity search - Function | |||||||||
| Biological species |   | |||||||||
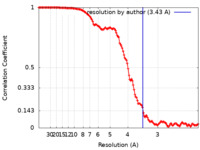
| Method | single particle reconstruction / cryo EM / Resolution: 3.43 Å | |||||||||
 Authors Authors | Pichkur EB / Stanishneva-Konovalova TB | |||||||||
| Funding support |  Russian Federation, 1 items Russian Federation, 1 items
| |||||||||
 Citation Citation |  Journal: Sci Rep / Year: 2021 Journal: Sci Rep / Year: 2021Title: Novel cryo-EM structure of an ADP-bound GroEL-GroES complex. Authors: Sofia S Kudryavtseva / Evgeny B Pichkur / Igor A Yaroshevich / Aleksandra A Mamchur / Irina S Panina / Andrei V Moiseenko / Olga S Sokolova / Vladimir I Muronetz / Tatiana B Stanishneva-Konovalova /  Abstract: The GroEL-GroES chaperonin complex is a bacterial protein folding system, functioning in an ATP-dependent manner. Upon ATP binding and hydrolysis, it undergoes multiple stages linked to substrate ...The GroEL-GroES chaperonin complex is a bacterial protein folding system, functioning in an ATP-dependent manner. Upon ATP binding and hydrolysis, it undergoes multiple stages linked to substrate protein binding, folding and release. Structural methods helped to reveal several conformational states and provide more information about the chaperonin functional cycle. Here, using cryo-EM we resolved two nucleotide-bound structures of the bullet-shaped GroEL-GroES complex at 3.4 Å resolution. The main difference between them is the relative orientation of their apical domains. Both structures contain nucleotides in cis and trans GroEL rings; in contrast to previously reported bullet-shaped complexes where nucleotides were only present in the cis ring. Our results suggest that the bound nucleotides correspond to ADP, and that such a state appears at low ATP:ADP ratios. | |||||||||
| History |
|
- Structure visualization
Structure visualization
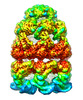
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_13308.map.gz emd_13308.map.gz | 8.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-13308-v30.xml emd-13308-v30.xml emd-13308.xml emd-13308.xml | 19.4 KB 19.4 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_13308_fsc.xml emd_13308_fsc.xml | 13.3 KB | Display |  FSC data file FSC data file |
| Images |  emd_13308.png emd_13308.png | 157.9 KB | ||
| Masks |  emd_13308_msk_1.map emd_13308_msk_1.map | 216 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-13308.cif.gz emd-13308.cif.gz | 6.1 KB | ||
| Others |  emd_13308_additional_1.map.gz emd_13308_additional_1.map.gz emd_13308_half_map_1.map.gz emd_13308_half_map_1.map.gz emd_13308_half_map_2.map.gz emd_13308_half_map_2.map.gz | 203.7 MB 200.6 MB 200.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-13308 http://ftp.pdbj.org/pub/emdb/structures/EMD-13308 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-13308 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-13308 | HTTPS FTP |
-Related structure data
| Related structure data |  7pbxMC  7pbjC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_13308.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_13308.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Locally sharpened C7 map of "tight" conformation of the GroEL-GroES complex. ADP bound to both rings. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.107 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
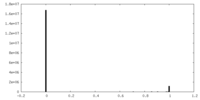
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_13308_msk_1.map emd_13308_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
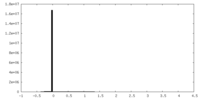
| Density Histograms |
-Additional map: Locally sharpened C1 map.
| File | emd_13308_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Locally sharpened C1 map. | ||||||||||||
| Projections & Slices |
| ||||||||||||
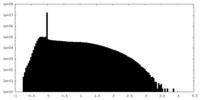
| Density Histograms |
-Half map: Half1
| File | emd_13308_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half2
| File | emd_13308_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
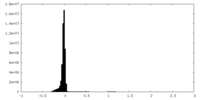
| Density Histograms |
- Sample components
Sample components
-Entire : ADP-bound GroEL-GroES complex
| Entire | Name: ADP-bound GroEL-GroES complex |
|---|---|
| Components |
|
-Supramolecule #1: ADP-bound GroEL-GroES complex
| Supramolecule | Name: ADP-bound GroEL-GroES complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #2 |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 882 KDa |
-Macromolecule #1: 60 kDa chaperonin
| Macromolecule | Name: 60 kDa chaperonin / type: protein_or_peptide / ID: 1 Details: GroEL from Escherichia coli str. K-12 substr. W3110 Number of copies: 14 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 55.220105 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: AAKDVKFGND ARVKMLRGVN VLADAVKVTL GPKGRNVVLD KSFGAPTITK DGVSVAREIE LEDKFENMGA QMVKEVASKA NDAAGDGTT TATVLAQAII TEGLKAVAAG MNPMDLKRGI DKAVTAAVEE LKALSVPCSD SKAIAQVGTI SANSDETVGK L IAEAMDKV ...String: AAKDVKFGND ARVKMLRGVN VLADAVKVTL GPKGRNVVLD KSFGAPTITK DGVSVAREIE LEDKFENMGA QMVKEVASKA NDAAGDGTT TATVLAQAII TEGLKAVAAG MNPMDLKRGI DKAVTAAVEE LKALSVPCSD SKAIAQVGTI SANSDETVGK L IAEAMDKV GKEGVITVED GTGLQDELDV VEGMQFDRGY LSPYFINKPE TGAVELESPF ILLADKKISN IREMLPVLEA VA KAGKPLL IIAEDVEGEA LATLVVNTMR GIVKVAAVKA PGFGDRRKAM LQDIATLTGG TVISEEIGME LEKATLEDLG QAK RVVINK DTTTIIDGVG EEAAIQGRVA QIRQQIEEAT SDYDREKLQE RVAKLAGGVA VIKVGAATEV EMKEKKARVE DALH ATRAA VEEGVVAGGG VALIRVASKL ADLRGQNEDQ NVGIKVALRA MEAPLRQIVL NCGEEPSVVA NTVKGGDGNY GYNAA TEEY GNMIDMGILD PTKVTRSALQ YAASVAGLMI TTECMVTDLP UniProtKB: Chaperonin GroEL |
-Macromolecule #2: 10 kDa chaperonin
| Macromolecule | Name: 10 kDa chaperonin / type: protein_or_peptide / ID: 2 Details: GroES from Escherichia coli str. K-12 substr. W3110 Number of copies: 7 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 10.400938 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MNIRPLHDRV IVKRKEVETK SAGGIVLTGS AAAKSTRGEV LAVGNGRILE NGEVKPLDVK VGDIVIFNDG YGVKSEKIDN EEVLIMSES DILAIVEA UniProtKB: Co-chaperonin GroES |
-Macromolecule #3: ADENOSINE-5'-DIPHOSPHATE
| Macromolecule | Name: ADENOSINE-5'-DIPHOSPHATE / type: ligand / ID: 3 / Number of copies: 14 / Formula: ADP |
|---|---|
| Molecular weight | Theoretical: 427.201 Da |
| Chemical component information |  ChemComp-ADP: |
-Macromolecule #4: MAGNESIUM ION
| Macromolecule | Name: MAGNESIUM ION / type: ligand / ID: 4 / Number of copies: 14 / Formula: MG |
|---|---|
| Molecular weight | Theoretical: 24.305 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY ARRAY |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 4.5 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON II (4k x 4k) / Detector mode: INTEGRATING / Average electron dose: 100.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 100.0 µm / Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD / Cs: 0.01 mm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller












 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)