+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | ABC Transporter complex NosDFYL, consensus refinement | |||||||||
 Map data Map data | unsharpened map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | ABC Transporter complex / metal-binding / ATP-free / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationABC-type transporter activity / periplasmic space / ATP hydrolysis activity / ATP binding / metal ion binding / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Pseudomonas stutzeri ATCC 14405 = CCUG 16156 (bacteria) Pseudomonas stutzeri ATCC 14405 = CCUG 16156 (bacteria) | |||||||||
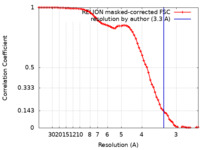
| Method | single particle reconstruction / cryo EM / Resolution: 3.3 Å | |||||||||
 Authors Authors | Mueller C / Zhang L | |||||||||
 Citation Citation |  Journal: Nature / Year: 2022 Journal: Nature / Year: 2022Title: Molecular interplay of an assembly machinery for nitrous oxide reductase. Authors: Christoph Müller / Lin Zhang / Sara Zipfel / Annika Topitsch / Marleen Lutz / Johannes Eckert / Benedikt Prasser / Mohamed Chami / Wei Lü / Juan Du / Oliver Einsle /    Abstract: Emissions of the critical ozone-depleting and greenhouse gas nitrous oxide (NO) from soils and industrial processes have increased considerably over the last decades. As the final step of bacterial ...Emissions of the critical ozone-depleting and greenhouse gas nitrous oxide (NO) from soils and industrial processes have increased considerably over the last decades. As the final step of bacterial denitrification, NO is reduced to chemically inert N (refs. ) in a reaction that is catalysed by the copper-dependent nitrous oxide reductase (NOR) (ref. ). The assembly of its unique [4Cu:2S] active site cluster Cu requires both the ATP-binding-cassette (ABC) complex NosDFY and the membrane-anchored copper chaperone NosL (refs. ). Here we report cryo-electron microscopy structures of Pseudomonas stutzeri NosDFY and its complexes with NosL and NOR, respectively. We find that the periplasmic NosD protein contains a binding site for a Cu ion and interacts specifically with NosL in its nucleotide-free state, whereas its binding to NOR requires a conformational change that is triggered by ATP binding. Mutually exclusive structures of NosDFY in complex with NosL and with NOR reveal a sequential metal-trafficking and assembly pathway for a highly complex copper site. Within this pathway, NosDFY acts as a mechanical energy transducer rather than as a transporter. It links ATP hydrolysis in the cytoplasm to a conformational transition of the NosD subunit in the periplasm, which is required for NosDFY to switch its interaction partner so that copper ions are handed over from the chaperone NosL to the enzyme NOR. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_13050.map.gz emd_13050.map.gz | 23.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-13050-v30.xml emd-13050-v30.xml emd-13050.xml emd-13050.xml | 17.6 KB 17.6 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_13050_fsc.xml emd_13050_fsc.xml | 7.2 KB | Display |  FSC data file FSC data file |
| Images |  emd_13050.png emd_13050.png | 35.4 KB | ||
| Filedesc metadata |  emd-13050.cif.gz emd-13050.cif.gz | 6 KB | ||
| Others |  emd_13050_additional_1.map.gz emd_13050_additional_1.map.gz | 28.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-13050 http://ftp.pdbj.org/pub/emdb/structures/EMD-13050 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-13050 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-13050 | HTTPS FTP |
-Related structure data
| Related structure data |  7osgMC  7o0yC  7o0zC  7o10C  7o11C  7o12C  7o13C  7o14C  7o15C  7o16C  7o17C  7osfC  7oshC  7osiC  7osjC  7qbaC  7znqC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_13050.map.gz / Format: CCP4 / Size: 30.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_13050.map.gz / Format: CCP4 / Size: 30.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | unsharpened map | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.296 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: sharpened map
| File | emd_13050_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | sharpened map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : ABC Transporter NosFY in complex with accessory periplasmic prote...
| Entire | Name: ABC Transporter NosFY in complex with accessory periplasmic protein NosD and Cu chaperone NosL |
|---|---|
| Components |
|
-Supramolecule #1: ABC Transporter NosFY in complex with accessory periplasmic prote...
| Supramolecule | Name: ABC Transporter NosFY in complex with accessory periplasmic protein NosD and Cu chaperone NosL type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#4 / Details: nucleotide-free state |
|---|---|
| Source (natural) | Organism:  Pseudomonas stutzeri ATCC 14405 = CCUG 16156 (bacteria) Pseudomonas stutzeri ATCC 14405 = CCUG 16156 (bacteria) |
-Macromolecule #1: Probable ABC transporter binding protein NosD
| Macromolecule | Name: Probable ABC transporter binding protein NosD / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Pseudomonas stutzeri ATCC 14405 = CCUG 16156 (bacteria) Pseudomonas stutzeri ATCC 14405 = CCUG 16156 (bacteria) |
| Molecular weight | Theoretical: 48.2585 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MFKAQATFSR YSAAVSLLLL FSGAAQAAPQ SITTLPLQPD GENRWRLPAG EYQGQFTIEQ PMQLRCEPGA VIQSQGQGSS LLISAPDVL VEGCTLYEWG SDLTAMDSAV FILPAAERAQ ISNNRMRGPG FGVFVDGTRD VQVIGNEIDG DAGVRSQDRG N GIHLFAVS ...String: MFKAQATFSR YSAAVSLLLL FSGAAQAAPQ SITTLPLQPD GENRWRLPAG EYQGQFTIEQ PMQLRCEPGA VIQSQGQGSS LLISAPDVL VEGCTLYEWG SDLTAMDSAV FILPAAERAQ ISNNRMRGPG FGVFVDGTRD VQVIGNEIDG DAGVRSQDRG N GIHLFAVS GARVLHNHVR NARDGIYIDT SNGNHLEGNV IEDVRYGVHY MFANENSLID NVTRRTRTGY ALMQSRKLTV TG NRSEQDQ NYGILMNYIT YSTITGNFVS DVQRGDTGGD SMISGGEGKA LFIYNSLFNT IENNHFEKSS LGIHLTAGSE DNR ISGNAF VGNQQQVKYV ASRTQEWSVD GRGNYWSDYL GWDRNNDGLG DIAYEPNDNV DRLLWLYPQV RLLMNSPSIE VLRW VQRAF PVIKSPGVQD SHPLMKLPTE KLLTEKQEPT S UniProtKB: Probable ABC transporter binding protein NosD |
-Macromolecule #2: Probable ABC transporter ATP-binding protein NosF
| Macromolecule | Name: Probable ABC transporter ATP-binding protein NosF / type: protein_or_peptide / ID: 2 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Pseudomonas stutzeri ATCC 14405 = CCUG 16156 (bacteria) Pseudomonas stutzeri ATCC 14405 = CCUG 16156 (bacteria) |
| Molecular weight | Theoretical: 33.821703 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MNAVEIQGVS QRYGSMTVLH DLNLNLGEGE VLGLFGHNGA GKTTSMKLIL GLLSPSEGQV KVLGRAPNDP QVRRQLGYLP ENVTFYPQL SGRETLRHFA RLKGAALTQV DELLEQVGLA HAADRRVKTY SKGMRQRLGL AQALLGEPRL LLLDEPTVGL D PIATQDLY ...String: MNAVEIQGVS QRYGSMTVLH DLNLNLGEGE VLGLFGHNGA GKTTSMKLIL GLLSPSEGQV KVLGRAPNDP QVRRQLGYLP ENVTFYPQL SGRETLRHFA RLKGAALTQV DELLEQVGLA HAADRRVKTY SKGMRQRLGL AQALLGEPRL LLLDEPTVGL D PIATQDLY LLIDRLRQRG TSIILCSHVL PGVEAHINRA AILAKGCLQA VGSLSQLRAE AGLPVRIRAS GISERDSWLQ RW TDAGHSA RGLSESSIEV VAVNGHKLVL LRQLLGEGEP EDIEIHQPSL EDLYRYYMER AGDVRAQEGR L UniProtKB: Probable ABC transporter ATP-binding protein NosF |
-Macromolecule #3: Probable ABC transporter permease protein NosY
| Macromolecule | Name: Probable ABC transporter permease protein NosY / type: protein_or_peptide / ID: 3 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Pseudomonas stutzeri ATCC 14405 = CCUG 16156 (bacteria) Pseudomonas stutzeri ATCC 14405 = CCUG 16156 (bacteria) |
| Molecular weight | Theoretical: 29.449203 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MNQVWNIARK ELSDGLRNRW LLAISLLFAV LAVGIAWLGA AASGQLGFTS IPATIASLAS LATFLMPLIA LLLAYDAIVG EDEGGTLML LLTYPLGRGQ ILLGKFVGHG LILALAVLIG FGCAALAIAL LVEGVELGML FWAFGRFMIS STLLGWVFLA F AYVLSGKV ...String: MNQVWNIARK ELSDGLRNRW LLAISLLFAV LAVGIAWLGA AASGQLGFTS IPATIASLAS LATFLMPLIA LLLAYDAIVG EDEGGTLML LLTYPLGRGQ ILLGKFVGHG LILALAVLIG FGCAALAIAL LVEGVELGML FWAFGRFMIS STLLGWVFLA F AYVLSGKV NEKSSAAGLA LGVWFLFVLV FDLVLLALLV LSEGKFNPEL LPWLLLLNPT DIYRLINLSG FEGSGSAMGV LS LGADLPV PAAVLWLCLL AWIGVSLLLA YAIFRRRLT UniProtKB: Probable ABC transporter permease protein NosY |
-Macromolecule #4: Copper-binding lipoprotein NosL
| Macromolecule | Name: Copper-binding lipoprotein NosL / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Pseudomonas stutzeri ATCC 14405 = CCUG 16156 (bacteria) Pseudomonas stutzeri ATCC 14405 = CCUG 16156 (bacteria) |
| Molecular weight | Theoretical: 20.47015 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MNALHRIGAG TLLAVLLAFG LTGCGEKEEV QQSLEPVAFH DSDECHVCGM IITDFPGPKG QAVEKRGVKK FCSTAEMLGW WLQPENRLL DAKLYVHDMG RSVWEKPDDG HLIDATSAYY VVGTSLKGAM GASLASFAEE QDAKALAGMH GGRVLRFEEI D QALLQEAA SMQHGGMHDH APNGAHNAHA GH UniProtKB: Copper-binding lipoprotein NosL |
-Macromolecule #5: MAGNESIUM ION
| Macromolecule | Name: MAGNESIUM ION / type: ligand / ID: 5 / Number of copies: 1 / Formula: MG |
|---|---|
| Molecular weight | Theoretical: 24.305 Da |
-Macromolecule #6: COPPER (II) ION
| Macromolecule | Name: COPPER (II) ION / type: ligand / ID: 6 / Number of copies: 1 / Formula: CU |
|---|---|
| Molecular weight | Theoretical: 63.546 Da |
| Chemical component information |  ChemComp-CU: |
-Macromolecule #7: ZINC ION
| Macromolecule | Name: ZINC ION / type: ligand / ID: 7 / Number of copies: 1 / Formula: ZN |
|---|---|
| Molecular weight | Theoretical: 65.409 Da |
-Macromolecule #8: water
| Macromolecule | Name: water / type: ligand / ID: 8 / Number of copies: 1 / Formula: HOH |
|---|---|
| Molecular weight | Theoretical: 18.015 Da |
| Chemical component information |  ChemComp-HOH: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 12 mg/mL |
|---|---|
| Buffer | pH: 8 |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average exposure time: 8.0 sec. / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller


























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)