+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-12438 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Mycobacterium smegmatis ATP synthase b-delta state 1 | |||||||||
 Map data Map data | M smegmatis ATP synthase bd-fusion combiset map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | complex / synthase / HYDROLASE | |||||||||
| Function / homology |  Function and homology information Function and homology informationproton-transporting ATPase activity, rotational mechanism / H+-transporting two-sector ATPase / proton-transporting ATP synthase complex / proton-transporting ATP synthase activity, rotational mechanism / ADP binding / ATP hydrolysis activity / ATP binding / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Mycolicibacterium smegmatis MC2 155 (bacteria) / Mycolicibacterium smegmatis MC2 155 (bacteria) /  Mycolicibacterium smegmatis (strain ATCC 700084 / mc(2)155) (bacteria) Mycolicibacterium smegmatis (strain ATCC 700084 / mc(2)155) (bacteria) | |||||||||
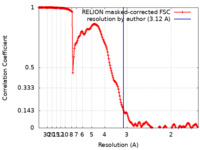
| Method | single particle reconstruction / cryo EM / Resolution: 3.12 Å | |||||||||
 Authors Authors | Montgomery MG / Petri J | |||||||||
| Funding support |  United Kingdom, 2 items United Kingdom, 2 items
| |||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2021 Journal: Proc Natl Acad Sci U S A / Year: 2021Title: Structure of the ATP synthase from provides targets for treating tuberculosis. Authors: Martin G Montgomery / Jessica Petri / Tobias E Spikes / John E Walker /  Abstract: The structure has been determined by electron cryomicroscopy of the adenosine triphosphate (ATP) synthase from This analysis confirms features in a prior description of the structure of the enzyme, ...The structure has been determined by electron cryomicroscopy of the adenosine triphosphate (ATP) synthase from This analysis confirms features in a prior description of the structure of the enzyme, but it also describes other highly significant attributes not recognized before that are crucial for understanding the mechanism and regulation of the mycobacterial enzyme. First, we resolved not only the three main states in the catalytic cycle described before but also eight substates that portray structural and mechanistic changes occurring during a 360° catalytic cycle. Second, a mechanism of auto-inhibition of ATP hydrolysis involves not only the engagement of the C-terminal region of an α-subunit in a loop in the γ-subunit, as proposed before, but also a "fail-safe" mechanism involving the b'-subunit in the peripheral stalk that enhances engagement. A third unreported characteristic is that the fused bδ-subunit contains a duplicated domain in its N-terminal region where the two copies of the domain participate in similar modes of attachment of the two of three N-terminal regions of the α-subunits. The auto-inhibitory plus the associated "fail-safe" mechanisms and the modes of attachment of the α-subunits provide targets for development of innovative antitubercular drugs. The structure also provides support for an observation made in the bovine ATP synthase that the transmembrane proton-motive force that provides the energy to drive the rotary mechanism is delivered directly and tangentially to the rotor via a Grotthuss water chain in a polar L-shaped tunnel. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_12438.map.gz emd_12438.map.gz | 10.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-12438-v30.xml emd-12438-v30.xml emd-12438.xml emd-12438.xml | 14.4 KB 14.4 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_12438_fsc.xml emd_12438_fsc.xml | 17.7 KB | Display |  FSC data file FSC data file |
| Images |  emd_12438.png emd_12438.png | 67.4 KB | ||
| Filedesc metadata |  emd-12438.cif.gz emd-12438.cif.gz | 6.1 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-12438 http://ftp.pdbj.org/pub/emdb/structures/EMD-12438 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-12438 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-12438 | HTTPS FTP |
-Related structure data
| Related structure data |  7nkdMC  7njkC  7njlC  7njmC  7njnC  7njoC  7njpC  7njqC  7njrC  7njsC  7njtC  7njuC  7njvC  7njwC  7njxC  7njyC  7nk7C  7nk9C  7nkbC  7nkhC  7nkjC  7nkkC  7nklC  7nknC  7nkpC  7nkqC  7nl9C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_12438.map.gz / Format: CCP4 / Size: 476.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_12438.map.gz / Format: CCP4 / Size: 476.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | M smegmatis ATP synthase bd-fusion combiset map | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.83 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Mycobacterium smegmatis ATP synthase
| Entire | Name: Mycobacterium smegmatis ATP synthase |
|---|---|
| Components |
|
-Supramolecule #1: Mycobacterium smegmatis ATP synthase
| Supramolecule | Name: Mycobacterium smegmatis ATP synthase / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Mycolicibacterium smegmatis MC2 155 (bacteria) Mycolicibacterium smegmatis MC2 155 (bacteria) |
| Molecular weight | Theoretical: 547 KDa |
-Macromolecule #1: ATP synthase subunit alpha
| Macromolecule | Name: ATP synthase subunit alpha / type: protein_or_peptide / ID: 1 / Number of copies: 3 / Enantiomer: LEVO / EC number: H+-transporting two-sector ATPase |
|---|---|
| Source (natural) | Organism:  Mycolicibacterium smegmatis (strain ATCC 700084 / mc(2)155) (bacteria) Mycolicibacterium smegmatis (strain ATCC 700084 / mc(2)155) (bacteria)Strain: ATCC 700084 / mc(2)155 |
| Molecular weight | Theoretical: 58.951461 KDa |
| Recombinant expression | Organism:  Mycolicibacterium smegmatis (bacteria) Mycolicibacterium smegmatis (bacteria) |
| Sequence | String: MAELTISAAD IEGAIEDYVS SFSADTEREE IGTVIDAGDG IAHVEGLPSV MTQELLEFPG GVLGVALNLD EHSVGAVILG EFEKIEEGQ QVKRTGEVLS VPVGDAFLGR VVNPLGQPID GQGDIAAETR RALELQAPSV VQRQSVSEPL QTGIKAIDAM T PIGRGQRQ ...String: MAELTISAAD IEGAIEDYVS SFSADTEREE IGTVIDAGDG IAHVEGLPSV MTQELLEFPG GVLGVALNLD EHSVGAVILG EFEKIEEGQ QVKRTGEVLS VPVGDAFLGR VVNPLGQPID GQGDIAAETR RALELQAPSV VQRQSVSEPL QTGIKAIDAM T PIGRGQRQ LIIGDRKTGK TAVCVDTILN QREAWLTGDP KQQVRCVYVA IGQKGTTIAS VKRALEEGGA MEYTTIVAAP AS DAAGFKW LAPYTGSAIG QHWMYNGKHV LIVFDDLSKQ ADAYRAISLL LRRPPGREAF PGDVFYLHSR LLERCAKLSD ELG GGSMTG LPIIETKAND ISAFIPTNVI SITDGQCFLE SDLFNQGVRP AINVGVSVSR VGGAAQIKAM KEVAGSLRLD LSQY RELEA FAAFASDLDA ASKAQLDRGA RLVELLKQPQ YSPLAVEEQV VAIFLGTQGH LDSVPVEDVQ RFESELLEHV KASHS DIFD GIRETKKLSE EAEEKLVSVI NEFKKGFQAS DGSSVVVSEN AEALDPEDLE KESVKVRKPA PKKA UniProtKB: ATP synthase subunit alpha |
-Macromolecule #2: ATP synthase subunit beta
| Macromolecule | Name: ATP synthase subunit beta / type: protein_or_peptide / ID: 2 Details: Poor alignment on Chain F there is no sequence conflict. DSLV is from res59, RGV is from res75 Number of copies: 3 / Enantiomer: LEVO / EC number: H+-transporting two-sector ATPase |
|---|---|
| Source (natural) | Organism:  Mycolicibacterium smegmatis (strain ATCC 700084 / mc(2)155) (bacteria) Mycolicibacterium smegmatis (strain ATCC 700084 / mc(2)155) (bacteria)Strain: ATCC 700084 / mc(2)155 |
| Molecular weight | Theoretical: 51.670453 KDa |
| Recombinant expression | Organism:  Mycolicibacterium smegmatis (bacteria) Mycolicibacterium smegmatis (bacteria) |
| Sequence | String: MTATAEKTAG RVVRITGPVV DVEFPRGSVP ELFNALHAEI TFGALAKTLT LEVAQHLGDS LVRCISMQPT DGLVRGVEVT DTGASISVP VGDGVKGHVF NALGDCLDDP GYGKDFEHWS IHRKPPAFSD LEPRTEMLET GLKVVDLLTP YVRGGKIALF G GAGVGKTV ...String: MTATAEKTAG RVVRITGPVV DVEFPRGSVP ELFNALHAEI TFGALAKTLT LEVAQHLGDS LVRCISMQPT DGLVRGVEVT DTGASISVP VGDGVKGHVF NALGDCLDDP GYGKDFEHWS IHRKPPAFSD LEPRTEMLET GLKVVDLLTP YVRGGKIALF G GAGVGKTV LIQEMINRIA RNFGGTSVFA GVGERTREGN DLWVELADAN VLKDTALVFG QMDEPPGTRM RVALSALTMA EF FRDEQGQ DVLLFIDNIF RFTQAGSEVS TLLGRMPSAV GYQPTLADEM GELQERITST RGRSITSMQA VYVPADDYTD PAP ATTFAH LDATTELSRA VFSKGIFPAV DPLASSSTIL DPAIVGDEHY RVAQEVIRIL QRYKDLQDII AILGIDELSE EDKQ LVNRA RRIERFLSQN MMAAEQFTGQ PGSTVPLKET IEAFDKLTKG EFDHLPEQAF FLIGGLDDLA KKAESLGAKL UniProtKB: ATP synthase subunit beta |
-Macromolecule #3: ATP synthase subunit b-delta
| Macromolecule | Name: ATP synthase subunit b-delta / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Mycolicibacterium smegmatis (strain ATCC 700084 / mc(2)155) (bacteria) Mycolicibacterium smegmatis (strain ATCC 700084 / mc(2)155) (bacteria)Strain: ATCC 700084 / mc(2)155 |
| Molecular weight | Theoretical: 47.504723 KDa |
| Recombinant expression | Organism:  Mycolicibacterium smegmatis (bacteria) Mycolicibacterium smegmatis (bacteria) |
| Sequence | String: MSIFIGQLIG FAVIAFIIVK WVVPPVRTLM RNQQEAVRAA LAESAEAAKK LADADAMHAK ALADAKAESE KVTEEAKQDS ERIAAQLSE QAGSEAERIK AQGAQQIQLM RQQLIRQLRT GLGAEAVNKA AEIVRAHVAD PQAQSATVDR FLSELEQMAP S SVVIDTAA ...String: MSIFIGQLIG FAVIAFIIVK WVVPPVRTLM RNQQEAVRAA LAESAEAAKK LADADAMHAK ALADAKAESE KVTEEAKQDS ERIAAQLSE QAGSEAERIK AQGAQQIQLM RQQLIRQLRT GLGAEAVNKA AEIVRAHVAD PQAQSATVDR FLSELEQMAP S SVVIDTAA TSRLRAASRQ SLAALVEKFD SVAGGLDADG LTNLADELAS VAKLLLSETA LNKHLAEPTD DSAPKVRLLE RL LSDKVSA TTLDLLRTAV SNRWSTESNL IDAVEHTARL ALLKRAEIAG EVDEVEEQLF RFGRVLDAEP RLSALLSDYT TPA EGRVAL LDKALTGRPG VNQTAAALLS QTVGLLRGER ADEAVIDLAE LAVSRRGEVV AHVSAAAELS DAQRTRLTEV LSRI YGRPV SVQLHVDPEL LGGLSITVGD EVIDGSIASR LAAAQTGLPD UniProtKB: ATP synthase subunit b-delta |
-Macromolecule #4: ATP synthase subunit b
| Macromolecule | Name: ATP synthase subunit b / type: protein_or_peptide / ID: 4 / Details: C-ter 10His tag / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Mycolicibacterium smegmatis (strain ATCC 700084 / mc(2)155) (bacteria) Mycolicibacterium smegmatis (strain ATCC 700084 / mc(2)155) (bacteria)Strain: ATCC 700084 / mc(2)155 |
| Molecular weight | Theoretical: 19.01817 KDa |
| Recombinant expression | Organism:  Mycolicibacterium smegmatis (bacteria) Mycolicibacterium smegmatis (bacteria) |
| Sequence | String: MGEFSATILA ASQAAEEGGG GSNFLIPNGT FFAVLIIFLI VLGVISKWVV PPISKVLAER EAMLAKTAAD NRKSAEQVAA AQADYEKEM AEARAQASAL RDEARAAGRS VVDEKRAQAS GEVAQTLTQA DQQLSAQGDQ VRSGLESSVD GLSAKLASRI L GVDVNSGG TQHHHHHHHH HH UniProtKB: ATP synthase subunit b |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 59.86 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller




































































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)