[English] 日本語
 Yorodumi
Yorodumi- EMDB-11961: Cryo-EM map of the membrane-associated light-dependent protochlor... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-11961 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM map of the membrane-associated light-dependent protochlorophyllide oxidoreductase (LPOR) from Z. mays (helical arrays, 16 units per turn) | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
| Biological species |  | |||||||||
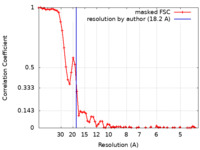
| Method | subtomogram averaging / cryo EM / Resolution: 18.2 Å | |||||||||
 Authors Authors | Floris D / Kuehlbrandt W | |||||||||
| Funding support |  Germany, 1 items Germany, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Plants / Year: 2021 Journal: Nat Plants / Year: 2021Title: Molecular landscape of etioplast inner membranes in higher plants. Authors: Davide Floris / Werner Kühlbrandt /  Abstract: Etioplasts are photosynthetically inactive plastids that accumulate when light levels are too low for chloroplast maturation. The etioplast inner membrane consists of a paracrystalline tubular ...Etioplasts are photosynthetically inactive plastids that accumulate when light levels are too low for chloroplast maturation. The etioplast inner membrane consists of a paracrystalline tubular lattice and peripheral, disk-shaped membranes, respectively known as the prolamellar body and prothylakoids. These distinct membrane regions are connected into one continuous compartment. To date, no structures of protein complexes in or at etioplast membranes have been reported. Here, we used electron cryo-tomography to explore the molecular membrane landscape of pea and maize etioplasts. Our tomographic reconstructions show that ATP synthase monomers are enriched in the prothylakoids, and plastid ribosomes in the tubular lattice. The entire tubular lattice is covered by regular helical arrays of a membrane-associated protein, which we identified as the 37-kDa enzyme, light-dependent protochlorophyllide oxidoreductase (LPOR). LPOR is the most abundant protein in the etioplast, where it is responsible for chlorophyll biosynthesis, photoprotection and defining the membrane geometry of the prolamellar body. Based on the 9-Å-resolution volume of the subtomogram average, we propose a structural model of membrane-associated LPOR. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_11961.map.gz emd_11961.map.gz | 20.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-11961-v30.xml emd-11961-v30.xml emd-11961.xml emd-11961.xml | 10.3 KB 10.3 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_11961_fsc.xml emd_11961_fsc.xml | 6.4 KB | Display |  FSC data file FSC data file |
| Images |  emd_11961.png emd_11961.png | 85.8 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-11961 http://ftp.pdbj.org/pub/emdb/structures/EMD-11961 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-11961 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-11961 | HTTPS FTP |
-Validation report
| Summary document |  emd_11961_validation.pdf.gz emd_11961_validation.pdf.gz | 339.6 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_11961_full_validation.pdf.gz emd_11961_full_validation.pdf.gz | 338.7 KB | Display | |
| Data in XML |  emd_11961_validation.xml.gz emd_11961_validation.xml.gz | 8.8 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-11961 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-11961 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-11961 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-11961 | HTTPS FTP |
-Related structure data
| Related structure data | C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_11961.map.gz / Format: CCP4 / Size: 22.2 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_11961.map.gz / Format: CCP4 / Size: 22.2 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 2.2 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : membrane-associated light-dependent protochlorophyllide oxidoredu...
| Entire | Name: membrane-associated light-dependent protochlorophyllide oxidoreductase (LPOR) from Z. mays (helical arrays, 16 units per turn) |
|---|---|
| Components |
|
-Supramolecule #1: membrane-associated light-dependent protochlorophyllide oxidoredu...
| Supramolecule | Name: membrane-associated light-dependent protochlorophyllide oxidoreductase (LPOR) from Z. mays (helical arrays, 16 units per turn) type: organelle_or_cellular_component / ID: 1 / Parent: 0 |
|---|---|
| Source (natural) | Organism:  |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | subtomogram averaging |
| Aggregation state | helical array |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Grid | Model: Quantifoil / Material: COPPER / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Average electron dose: 2.2 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: BRIGHT FIELD |
| Sample stage | Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller









 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)