+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-11585 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
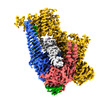
| Title | Cryo-EM structure of S.cerevisiae cohesin-Scc2-DNA complex | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | SMC / cohesin / DNA / CELL CYCLE | |||||||||
| Function / homology |  Function and homology information Function and homology informationSMC loading complex / Scc2-Scc4 cohesin loading complex / 2-micrometer circle DNA / Establishment of Sister Chromatid Cohesion / Resolution of Sister Chromatid Cohesion / cohesin loader activity / tRNA gene clustering / DNA secondary structure binding / meiotic cohesin complex / establishment of meiotic sister chromatid cohesion ...SMC loading complex / Scc2-Scc4 cohesin loading complex / 2-micrometer circle DNA / Establishment of Sister Chromatid Cohesion / Resolution of Sister Chromatid Cohesion / cohesin loader activity / tRNA gene clustering / DNA secondary structure binding / meiotic cohesin complex / establishment of meiotic sister chromatid cohesion / cohesin complex / mitotic cohesin complex / rDNA chromatin condensation / transcription-dependent tethering of RNA polymerase II gene DNA at nuclear periphery / synaptonemal complex assembly / establishment of protein localization to chromatin / SUMOylation of DNA damage response and repair proteins / meiotic sister chromatid cohesion / replication-born double-strand break repair via sister chromatid exchange / establishment of mitotic sister chromatid cohesion / mitotic chromosome condensation / chromatin looping / reciprocal meiotic recombination / sister chromatid cohesion / mitotic sister chromatid cohesion / protein acetylation / minor groove of adenine-thymine-rich DNA binding / mitotic sister chromatid segregation / chromosome, centromeric region / protein localization to chromatin / condensed nuclear chromosome / G2/M transition of mitotic cell cycle / double-strand break repair / regulation of gene expression / double-stranded DNA binding / sequence-specific DNA binding / cell division / apoptotic process / DNA damage response / chromatin binding / protein kinase binding / chromatin / ATP hydrolysis activity / mitochondrion / DNA binding / ATP binding / identical protein binding / nucleus / cytosol Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.4 Å | |||||||||
 Authors Authors | Lee B-G / Gonzalez Llamazares A | |||||||||
| Funding support |  United Kingdom, 2 items United Kingdom, 2 items
| |||||||||
 Citation Citation |  Journal: Elife / Year: 2020 Journal: Elife / Year: 2020Title: Transport of DNA within cohesin involves clamping on top of engaged heads by Scc2 and entrapment within the ring by Scc3. Authors: James E Collier / Byung-Gil Lee / Maurici Brunet Roig / Stanislav Yatskevich / Naomi J Petela / Jean Metson / Menelaos Voulgaris / Andres Gonzalez Llamazares / Jan Löwe / Kim A Nasmyth /  Abstract: In addition to extruding DNA loops, cohesin entraps within its SMC-kleisin ring (S-K) individual DNAs during G1 and sister DNAs during S-phase. All three activities require related hook-shaped ...In addition to extruding DNA loops, cohesin entraps within its SMC-kleisin ring (S-K) individual DNAs during G1 and sister DNAs during S-phase. All three activities require related hook-shaped proteins called Scc2 and Scc3. Using thiol-specific crosslinking we provide rigorous proof of entrapment activity in vitro. Scc2 alone promotes entrapment of DNAs in the E-S and E-K compartments, between ATP-bound engaged heads and the SMC hinge and associated kleisin, respectively. This does not require ATP hydrolysis nor is it accompanied by entrapment within S-K rings, which is a slower process requiring Scc3. Cryo-EM reveals that DNAs transported into E-S/E-K compartments are 'clamped' in a sub-compartment created by Scc2's association with engaged heads whose coiled coils are folded around their elbow. We suggest that clamping may be a recurrent feature of cohesin complexes active in loop extrusion and that this conformation precedes the S-K entrapment required for sister chromatid cohesion. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_11585.map.gz emd_11585.map.gz | 8.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-11585-v30.xml emd-11585-v30.xml emd-11585.xml emd-11585.xml | 27 KB 27 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_11585.png emd_11585.png | 147.5 KB | ||
| Masks |  emd_11585_msk_1.map emd_11585_msk_1.map | 125 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-11585.cif.gz emd-11585.cif.gz | 7.7 KB | ||
| Others |  emd_11585_half_map_1.map.gz emd_11585_half_map_1.map.gz emd_11585_half_map_2.map.gz emd_11585_half_map_2.map.gz | 98.5 MB 98.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-11585 http://ftp.pdbj.org/pub/emdb/structures/EMD-11585 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-11585 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-11585 | HTTPS FTP |
-Related structure data
| Related structure data |  6zz6MC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_11585.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_11585.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.07 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_11585_msk_1.map emd_11585_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_11585_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_11585_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
+Entire : Complex of cohesin, Scc2, ATP and DNA
+Supramolecule #1: Complex of cohesin, Scc2, ATP and DNA
+Supramolecule #2: Complex of cohesin and Scc2
+Supramolecule #3: double strand DNA
+Macromolecule #1: Structural maintenance of chromosomes protein 1,Structural mainte...
+Macromolecule #2: Structural maintenance of chromosomes protein 3,Structural mainte...
+Macromolecule #3: Sister chromatid cohesion protein 1,Sister chromatid cohesion pro...
+Macromolecule #4: Sister chromatid cohesion protein 2
+Macromolecule #5: DNA (34-MER)
+Macromolecule #6: DNA (34-MER)
+Macromolecule #7: ADENOSINE-5'-TRIPHOSPHATE
+Macromolecule #8: MAGNESIUM ION
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.3 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Grid | Model: UltrAuFoil / Material: GOLD / Mesh: 200 |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 100 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 55.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 3.0 µm / Nominal defocus min: 1.5 µm / Nominal magnification: 81000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: INSILICO MODEL |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.4 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 588164 |
| Initial angle assignment | Type: ANGULAR RECONSTITUTION |
| Final angle assignment | Type: ANGULAR RECONSTITUTION |
 Movie
Movie Controller
Controller











 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)