+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-11380 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | amyloid structure of amylin (IAPP - islet amyloid polypeptide) | |||||||||
 Map data Map data | post-processed-masked map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | amyloid fibril type-2-diabetes hormone / PROTEIN FIBRIL | |||||||||
| Function / homology |  Function and homology information Function and homology informationamylin receptor 3 signaling pathway / amylin receptor 2 signaling pathway / amylin receptor 1 signaling pathway / amylin receptor signaling pathway / Calcitonin-like ligand receptors / negative regulation of amyloid fibril formation / negative regulation of bone resorption / eating behavior / negative regulation of osteoclast differentiation / Regulation of gene expression in beta cells ...amylin receptor 3 signaling pathway / amylin receptor 2 signaling pathway / amylin receptor 1 signaling pathway / amylin receptor signaling pathway / Calcitonin-like ligand receptors / negative regulation of amyloid fibril formation / negative regulation of bone resorption / eating behavior / negative regulation of osteoclast differentiation / Regulation of gene expression in beta cells / positive regulation of cAMP/PKA signal transduction / bone resorption / negative regulation of protein-containing complex assembly / sensory perception of pain / positive regulation of calcium-mediated signaling / osteoclast differentiation / hormone activity / cell-cell signaling / amyloid-beta binding / G alpha (s) signalling events / positive regulation of MAPK cascade / positive regulation of apoptotic process / Amyloid fiber formation / receptor ligand activity / signaling receptor binding / neuronal cell body / apoptotic process / lipid binding / signal transduction / extracellular space / extracellular region / identical protein binding Similarity search - Function | |||||||||
| Biological species | synthetic construct (others) | |||||||||
| Method | helical reconstruction / cryo EM / Resolution: 3.6 Å | |||||||||
 Authors Authors | Gallardo RU / Iadanza MG | |||||||||
| Funding support |  United Kingdom, 2 items United Kingdom, 2 items
| |||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2020 Journal: Nat Struct Mol Biol / Year: 2020Title: Fibril structures of diabetes-related amylin variants reveal a basis for surface-templated assembly. Authors: Rodrigo Gallardo / Matthew G Iadanza / Yong Xu / George R Heath / Richard Foster / Sheena E Radford / Neil A Ranson /  Abstract: Aggregation of the peptide hormone amylin into amyloid deposits is a pathological hallmark of type-2 diabetes (T2D). While no causal link between T2D and amyloid has been established, the S20G ...Aggregation of the peptide hormone amylin into amyloid deposits is a pathological hallmark of type-2 diabetes (T2D). While no causal link between T2D and amyloid has been established, the S20G mutation in amylin is associated with early-onset T2D. Here we report cryo-EM structures of amyloid fibrils of wild-type human amylin and its S20G variant. The wild-type fibril structure, solved to 3.6-Å resolution, contains two protofilaments, each built from S-shaped subunits. S20G fibrils, by contrast, contain two major polymorphs. Their structures, solved at 3.9-Å and 4.0-Å resolution, respectively, share a common two-protofilament core that is distinct from the wild-type structure. Remarkably, one polymorph contains a third subunit with another, distinct, cross-β conformation. The presence of two different backbone conformations within the same fibril may explain the increased aggregation propensity of S20G, and illustrates a potential structural basis for surface-templated fibril assembly. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_11380.map.gz emd_11380.map.gz | 1.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-11380-v30.xml emd-11380-v30.xml emd-11380.xml emd-11380.xml | 23.6 KB 23.6 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_11380.png emd_11380.png | 49.5 KB | ||
| Masks |  emd_11380_msk_1.map emd_11380_msk_1.map | 30.5 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-11380.cif.gz emd-11380.cif.gz | 6 KB | ||
| Others |  emd_11380_additional_1.map.gz emd_11380_additional_1.map.gz emd_11380_additional_2.map.gz emd_11380_additional_2.map.gz emd_11380_half_map_1.map.gz emd_11380_half_map_1.map.gz emd_11380_half_map_2.map.gz emd_11380_half_map_2.map.gz | 23.4 MB 28.1 MB 23.4 MB 23.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-11380 http://ftp.pdbj.org/pub/emdb/structures/EMD-11380 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-11380 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-11380 | HTTPS FTP |
-Related structure data
| Related structure data |  6zrfMC  6zrqC  6zrrC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_11380.map.gz / Format: CCP4 / Size: 30.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_11380.map.gz / Format: CCP4 / Size: 30.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | post-processed-masked map | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.065 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
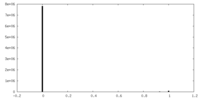
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_11380_msk_1.map emd_11380_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
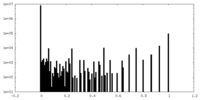
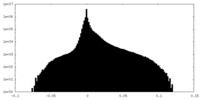
| Density Histograms |
-Additional map: 3D-auto-refined map
| File | emd_11380_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | 3D-auto-refined map | ||||||||||||
| Projections & Slices |
| ||||||||||||
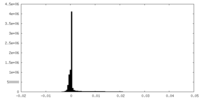
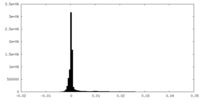
| Density Histograms |
-Additional map: post-processed not-masked map
| File | emd_11380_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | post-processed not-masked map | ||||||||||||
| Projections & Slices |
| ||||||||||||
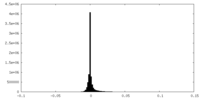
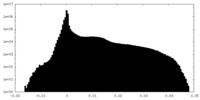
| Density Histograms |
-Half map: 3D-auto-refined half1 map
| File | emd_11380_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | 3D-auto-refined half1 map | ||||||||||||
| Projections & Slices |
| ||||||||||||
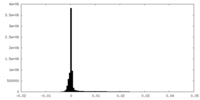
| Density Histograms |
-Half map: 3D-auto-refined half2 map
| File | emd_11380_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | 3D-auto-refined half2 map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : amyloid fibril of amylin (islet amyloid polipeptide)
| Entire | Name: amyloid fibril of amylin (islet amyloid polipeptide) |
|---|---|
| Components |
|
-Supramolecule #1: amyloid fibril of amylin (islet amyloid polipeptide)
| Supramolecule | Name: amyloid fibril of amylin (islet amyloid polipeptide) / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all Details: produced synthetically, oxidised, C-terminally amidated, fibrillated in vitro |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 1.625 kDa/nm |
-Macromolecule #1: Islet amyloid polypeptide
| Macromolecule | Name: Islet amyloid polypeptide / type: protein_or_peptide / ID: 1 / Details: TYC = C-terminal amidated Tyr / Number of copies: 12 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 3.908319 KDa |
| Sequence | String: KCNTATCATQ RLANFLVHSS NNFGAILSST NVGSNT(TYC) UniProtKB: Islet amyloid polypeptide |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | helical reconstruction |
| Aggregation state | helical array |
- Sample preparation
Sample preparation
| Concentration | 0.18 mg/mL |
|---|---|
| Buffer | pH: 6.8 / Component - Concentration: 20.0 mM / Component - Formula: NH4CH3CO2 / Component - Name: Ammonium acetate |
| Grid | Model: Homemade / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: LACEY / Pretreatment - Type: PLASMA CLEANING / Pretreatment - Time: 60 sec. |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 95 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Quantum LS / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: INTEGRATING / Average exposure time: 14.0 sec. / Average electron dose: 50.07 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Final reconstruction | Applied symmetry - Helical parameters - Δz: 2.43 Å Applied symmetry - Helical parameters - Δ&Phi: 178.23 ° Applied symmetry - Helical parameters - Axial symmetry: C1 (asymmetric) Resolution.type: BY AUTHOR / Resolution: 3.6 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 32846 |
|---|---|
| Startup model | Type of model: INSILICO MODEL |
| Final angle assignment | Type: NOT APPLICABLE |
 Movie
Movie Controller
Controller




















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)