+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-10325 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | cryo-EM map of ASFV MCP p72 homotrimers purified from virion | ||||||||||||
 Map data Map data | P72 postprocess map | ||||||||||||
 Sample Sample |
| ||||||||||||
| Biological species |   African swine fever virus African swine fever virus | ||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 4.6 Å | ||||||||||||
 Authors Authors | Abrescia NG / Andres G / Charro D | ||||||||||||
| Funding support |  Spain, 3 items Spain, 3 items
| ||||||||||||
 Citation Citation |  Journal: J Biol Chem / Year: 2020 Journal: J Biol Chem / Year: 2020Title: The cryo-EM structure of African swine fever virus unravels a unique architecture comprising two icosahedral protein capsids and two lipoprotein membranes. Authors: German Andrés / Diego Charro / Tania Matamoros / Rebecca S Dillard / Nicola G A Abrescia /   Abstract: African swine fever virus (ASFV) is a complex nucleocytoplasmic large DNA virus (NCLDV) that causes a devastating swine disease currently present in many countries of Africa, Europe, and Asia. ...African swine fever virus (ASFV) is a complex nucleocytoplasmic large DNA virus (NCLDV) that causes a devastating swine disease currently present in many countries of Africa, Europe, and Asia. Despite intense research efforts, relevant gaps in the architecture of the infectious virus particle remain. Here, we used single-particle cryo-EM to analyze the three-dimensional structure of the mature ASFV particle. Our results show that the ASFV virion, with a radial diameter of ∼2,080 Å, encloses a genome-containing nucleoid surrounded by two distinct icosahedral protein capsids and two lipoprotein membranes. The outer capsid forms a hexagonal lattice (triangulation number = 277) composed of 8,280 copies of the double jelly-roll major capsid protein (MCP) p72, arranged in trimers displaying a pseudo-hexameric morphology, and of 60 copies of a penton protein at the vertices. The inner protein layer, organized as a = 19 capsid, confines the core shell, and it is composed of the mature products derived from the ASFV polyproteins pp220 and pp62. Also, an icosahedral membrane lies between the two protein layers, whereas a pleomorphic envelope wraps the outer capsid. This high-level organization confers to ASFV a unique architecture among the NCLDVs that likely reflects the complexity of its infection process and may help explain current challenges in controlling it. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_10325.map.gz emd_10325.map.gz | 25.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-10325-v30.xml emd-10325-v30.xml emd-10325.xml emd-10325.xml | 20.7 KB 20.7 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_10325_fsc.xml emd_10325_fsc.xml | 6.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_10325.png emd_10325.png | 104 KB | ||
| Masks |  emd_10325_msk_1.map emd_10325_msk_1.map | 27 MB |  Mask map Mask map | |
| Others |  emd_10325_additional_1.map.gz emd_10325_additional_1.map.gz emd_10325_additional_2.map.gz emd_10325_additional_2.map.gz emd_10325_half_map_1.map.gz emd_10325_half_map_1.map.gz emd_10325_half_map_2.map.gz emd_10325_half_map_2.map.gz | 18 MB 14.3 MB 20.5 MB 20.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-10325 http://ftp.pdbj.org/pub/emdb/structures/EMD-10325 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-10325 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-10325 | HTTPS FTP |
-Validation report
| Summary document |  emd_10325_validation.pdf.gz emd_10325_validation.pdf.gz | 377.1 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_10325_full_validation.pdf.gz emd_10325_full_validation.pdf.gz | 376.2 KB | Display | |
| Data in XML |  emd_10325_validation.xml.gz emd_10325_validation.xml.gz | 12.7 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-10325 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-10325 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-10325 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-10325 | HTTPS FTP |
-Related structure data
| Related structure data | C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_10325.map.gz / Format: CCP4 / Size: 27 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_10325.map.gz / Format: CCP4 / Size: 27 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | P72 postprocess map | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.13 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_10325_msk_1.map emd_10325_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
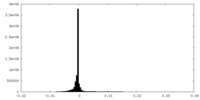
| Density Histograms |
-Additional map: local resolution filtered map
| File | emd_10325_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | local resolution filtered map | ||||||||||||
| Projections & Slices |
| ||||||||||||
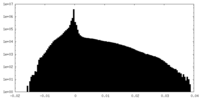
| Density Histograms |
-Additional map: for coloring locres
| File | emd_10325_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | for coloring locres | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: P72 Half1
| File | emd_10325_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | P72 Half1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
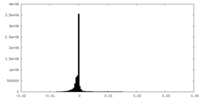
| Density Histograms |
-Half map: P72 Half2
| File | emd_10325_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | P72 Half2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
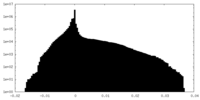
| Density Histograms |
- Sample components
Sample components
-Entire : homotrimer of Major Capsid Protein p72 of African Swine Fever virus
| Entire | Name: homotrimer of Major Capsid Protein p72 of African Swine Fever virus |
|---|---|
| Components |
|
-Supramolecule #1: homotrimer of Major Capsid Protein p72 of African Swine Fever virus
| Supramolecule | Name: homotrimer of Major Capsid Protein p72 of African Swine Fever virus type: complex / ID: 1 / Parent: 0 / Macromolecule list: all / Details: purified from virion |
|---|---|
| Source (natural) | Organism:   African swine fever virus / Strain: Ba71V African swine fever virus / Strain: Ba71V |
-Macromolecule #1: viral major capsid protein p72
| Macromolecule | Name: viral major capsid protein p72 / type: protein_or_peptide / ID: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   African swine fever virus / Strain: strain Badajoz 1971 African swine fever virus / Strain: strain Badajoz 1971 |
| Sequence | String: MASGGAFCLI ANDGKADKII LAQDLLNSRI SNIKNVNKSY GKPDPEPTLS QIEETHLVHF NAHFKPYVP VGFEYNKVRP HTGTPTLGNK LTFGIPQYGD FFHDMVGHHI LGACHSSWQD A PIQGTAQM GAHGQLQTFP RNGYDWDNQT PLEGAVYTLV DPFGRPIVPG ...String: MASGGAFCLI ANDGKADKII LAQDLLNSRI SNIKNVNKSY GKPDPEPTLS QIEETHLVHF NAHFKPYVP VGFEYNKVRP HTGTPTLGNK LTFGIPQYGD FFHDMVGHHI LGACHSSWQD A PIQGTAQM GAHGQLQTFP RNGYDWDNQT PLEGAVYTLV DPFGRPIVPG TKNAYRNLVY YC EYPGERL YENVRFDVNG NSLDEYSSDV TTLVRKFCIP GDKMTGYKHL VGQEVSVEGT SGP LLCNIH DLHKPHQSKP ILTDENDTQR TCSHTNPKFL SQHFPENSHN IQTAGKQDIT PITD ATYLD IRRNVHYSCN GPQTPKYYQP PLALWIKLRF WFNENVNLAI PSVSIPFGER FITIK LASQ KDLVNEFPGL FIRQSRFIPG RPSRRNIRFK PWFIPGVINE ISLTNNELYI NNLFVT PEI HNLFVKRVRF SLIRVHKTQV THTNNNHHDE KLMSALKWPI EYMFIGLKPT WNISDQN PH QHRDWHKFGH VVNAIMQPTH HAEISFQDRD TALPDACSSI SDISPVTYPI TLPIIKNI S VTAHGINLID KFPSKFCSSY IPFHYGGNAI KTPDDPGAMM ITFALKPREE YQPSGHINV SRAREFYISW DTDYVGSITT ADLVVSASAI NFLLLQNGSA VLRYST |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1.1 mg/mL |
|---|---|
| Buffer | pH: 7.4 / Component - Name: PBS |
| Grid | Model: Quantifoil R2/2 / Material: COPPER / Mesh: 300 / Support film - Material: GRAPHENE OXIDE / Support film - topology: HOLEY |
| Vitrification | Cryogen name: ETHANE-PROPANE / Chamber humidity: 90 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK III |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Detector mode: COUNTING / Number grids imaged: 1 / Number real images: 1541 / Average electron dose: 46.6 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: DARK FIELD / Cs: 0.01 mm / Nominal magnification: 59000 |
| Sample stage | Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller











 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)