+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 | 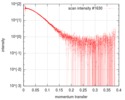 |
|---|---|
 試料 試料 | Aryl-hydrocarbon-interacting protein-like 1
|
| 機能・相同性 |  機能・相同性情報 機能・相同性情報farnesylated protein binding / protein farnesylation / regulation of opsin-mediated signaling pathway / retina homeostasis / phototransduction, visible light / photoreceptor inner segment / visual perception / peptidyl-prolyl cis-trans isomerase activity / unfolded protein binding / nuclear speck ...farnesylated protein binding / protein farnesylation / regulation of opsin-mediated signaling pathway / retina homeostasis / phototransduction, visible light / photoreceptor inner segment / visual perception / peptidyl-prolyl cis-trans isomerase activity / unfolded protein binding / nuclear speck / apoptotic process / negative regulation of apoptotic process / nucleoplasm / nucleus / cytosol / cytoplasm 類似検索 - 分子機能 |
| 生物種 |  Homo sapiens (ヒト) Homo sapiens (ヒト) |
 引用 引用 |  ジャーナル: J Biol Chem / 年: 2019 ジャーナル: J Biol Chem / 年: 2019タイトル: Interaction of the tetratricopeptide repeat domain of aryl hydrocarbon receptor-interacting protein-like 1 with the regulatory Pγ subunit of phosphodiesterase 6. 著者: Ravi P Yadav / Kimberly Boyd / Liping Yu / Nikolai O Artemyev /  要旨: Phosphodiesterase-6 (PDE6) is key to both phototransduction and health of rods and cones. Proper folding of PDE6 relies on the chaperone activity of aryl hydrocarbon receptor-interacting protein-like ...Phosphodiesterase-6 (PDE6) is key to both phototransduction and health of rods and cones. Proper folding of PDE6 relies on the chaperone activity of aryl hydrocarbon receptor-interacting protein-like 1 (AIPL1), and mutations in both PDE6 and AIPL1 can cause a severe form of blindness. Although AIPL1 and PDE6 are known to interact via the FK506-binding protein domain of AIPL1, the contribution of the tetratricopeptide repeat (TPR) domain of AIPL1 to its chaperone function is poorly understood. Here, we demonstrate that AIPL1-TPR interacts specifically with the regulatory Pγ subunit of PDE6. Use of NMR chemical shift perturbation (CSP) mapping technique revealed the interface between the C-terminal portion of Pγ and AIPL1-TPR. Our solution of the crystal structure of the AIPL1-TPR domain provided additional information, which together with the CSP data enabled us to generate a model of this interface. Biochemical analysis of chimeric AIPL1-AIP proteins supported this model and also revealed a correlation between the affinity of AIPL1-TPR for Pγ and the ability of Pγ to potentiate the chaperone activity of AIPL1. Based on these results, we present a model of the larger AIPL1-PDE6 complex. This supports the importance of simultaneous interactions of AIPL1-FK506-binding protein with the prenyl moieties of PDE6 and AIPL1-TPR with the Pγ subunit during the folding and/or assembly of PDE6. This study sheds new light on the versatility of TPR domains in protein folding by describing a novel TPR-protein binding partner, Pγ, and revealing that this subunit imparts AIPL1 selectivity for its client. |
 登録者 登録者 |
|
- 構造の表示
構造の表示
- ダウンロードとリンク
ダウンロードとリンク
-Data source
| SASBDBのページ |  SASDGX4 SASDGX4 |
|---|
-関連構造データ
| 関連構造データ |  6px0C C: 同じ文献を引用 ( |
|---|---|
| 類似構造データ | 類似検索 - 機能・相同性  F&H 検索 F&H 検索 |
- 外部リンク
外部リンク
| 「今月の分子」の関連する項目 |
|---|
-モデル
- 試料
試料
 試料 試料 | 名称: Aryl-hydrocarbon-interacting protein-like 1 / 試料濃度: 10 mg/ml |
|---|---|
| バッファ | 名称: 50 mM Tris, 100 mM NaCl, 2.5 % glycerol and 6 mM DTT pH: 7.5 |
| 要素 #1870 | 名称: AIPL1 / タイプ: protein / 記述: Aryl-hydrocarbon-interacting protein-like 1(1-316) / 分子量: 37.105 / 分子数: 1 / 由来: Homo sapiens / 参照: UniProt: Q9NZN9 配列: SHMDAALLLN VEGVKKTILH GGTGELPNFI TGSRVIFHFR TMKCDEERTV IDDSRQVGQP MHIIIGNMFK LEVWEILLTS MRVHEVAEFW CDTIHTGVYP ILSRSLRQMA QGKDPTEWHV HTCGLANMFA YHTLGYEDLD ELQKEPQPLV FVIELLQVDA PSDYQRETWN ...配列: SHMDAALLLN VEGVKKTILH GGTGELPNFI TGSRVIFHFR TMKCDEERTV IDDSRQVGQP MHIIIGNMFK LEVWEILLTS MRVHEVAEFW CDTIHTGVYP ILSRSLRQMA QGKDPTEWHV HTCGLANMFA YHTLGYEDLD ELQKEPQPLV FVIELLQVDA PSDYQRETWN LSNHEKMKAV PVLHGEGNRL FKLGRYEEAS SKYQEAIICL RNLQTKEKPW EVQWLKLEKM INTLILNYCQ CLLKKEEYYE VLEHTSDILR HHPGIVKAYY VRARAHAEVW NEAEAKADLQ KVLELEPSMQ KAVRRELRLL ENRMAEKQ |
-実験情報
| ビーム | 設備名称: Advanced Photon Source (APS), Argonne National Laboratory BioCAT 18ID 地域: Lemont, IL / 国: USA  / 線源: X-ray synchrotron / 波長: 0.1033 Å / スペクトロメータ・検出器間距離: 3.5 mm / 線源: X-ray synchrotron / 波長: 0.1033 Å / スペクトロメータ・検出器間距離: 3.5 mm | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 検出器 | 名称: Pilatus3 X 1M / Pixsize x: 0.172 mm | ||||||||||||||||||||||||||||||
| スキャン |
| ||||||||||||||||||||||||||||||
| 距離分布関数 P(R) |
| ||||||||||||||||||||||||||||||
| 結果 |
|
 ムービー
ムービー コントローラー
コントローラー






