[English] 日本語
 Yorodumi
Yorodumi- SASDF35: R16-24 del45-51 human dystrophin fragment (Human dystrophin centr... -
+ Open data
Open data
- Basic information
Basic information
| Entry | 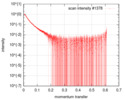 |
|---|---|
 Sample Sample | R16-24 del45-51 human dystrophin fragment
|
| Function / homology |  Function and homology information Function and homology informationregulation of muscle system process / regulation of cellular response to growth factor stimulus / syntrophin complex / cardiac muscle cell action potential / regulation of skeletal muscle contraction / dystrophin-associated glycoprotein complex / synaptic signaling / cell-substrate junction / peptide biosynthetic process / motile cilium assembly ...regulation of muscle system process / regulation of cellular response to growth factor stimulus / syntrophin complex / cardiac muscle cell action potential / regulation of skeletal muscle contraction / dystrophin-associated glycoprotein complex / synaptic signaling / cell-substrate junction / peptide biosynthetic process / motile cilium assembly / dystroglycan binding / regulation of skeletal muscle contraction by regulation of release of sequestered calcium ion / vinculin binding / regulation of sodium ion transmembrane transport / Formation of the dystrophin-glycoprotein complex (DGC) / costamere / muscle cell development / regulation of calcium ion transmembrane transport / neuron projection terminus / Striated Muscle Contraction / filopodium membrane / structural constituent of muscle / muscle organ development / muscle cell cellular homeostasis / myosin binding / maintenance of blood-brain barrier / nitric-oxide synthase binding / Non-integrin membrane-ECM interactions / neuron development / regulation of cardiac muscle contraction by regulation of the release of sequestered calcium ion / skeletal muscle tissue development / cardiac muscle contraction / regulation of release of sequestered calcium ion into cytosol by sarcoplasmic reticulum / response to muscle stretch / positive regulation of neuron differentiation / regulation of heart rate / filopodium / positive regulation of neuron projection development / sarcolemma / structural constituent of cytoskeleton / Z disc / intracellular protein localization / actin binding / protein-containing complex assembly / postsynaptic membrane / cytoskeleton / membrane raft / synapse / cell surface / protein-containing complex / zinc ion binding / nucleus / plasma membrane / cytosol Similarity search - Function |
 Contact author Contact author |
|
- Structure visualization
Structure visualization
- Downloads & links
Downloads & links
-Data source
| SASBDB page |  SASDF35 SASDF35 |
|---|
-Related structure data
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
|---|
- External links
External links
| Related items in Molecule of the Month |
|---|
-Models
- Sample
Sample
 Sample Sample | Name: R16-24 del45-51 human dystrophin fragment |
|---|---|
| Buffer | Name: NaP 20 mM, NaCl 300 mM, EDTA 1 mM, Glycérol 2% / pH: 7.5 |
| Entity #1562 | Type: protein Description: Human dystrophin central domain R16-24 del45-51 fragment Formula weight: 84.61 / Num. of mol.: 1 / References: UniProt: P11532 Sequence: MSYYHHHHHH DYDIPTTENL YFOGAMGRSL VPRGSLEISY VPSTYLTEIT HVSQALLEVE QLLNAPDLCA KDFEDLFKQE ESLKNIKDSL QQSSGRIDII HSKKTAALQS ATPVERVKLQ EALSQLDFQW EKVNKMYKDR QGRFDRSVEK WRRFHYDIKI FNQWLTEAEQ ...Sequence: MSYYHHHHHH DYDIPTTENL YFOGAMGRSL VPRGSLEISY VPSTYLTEIT HVSQALLEVE QLLNAPDLCA KDFEDLFKQE ESLKNIKDSL QQSSGRIDII HSKKTAALQS ATPVERVKLQ EALSQLDFQW EKVNKMYKDR QGRFDRSVEK WRRFHYDIKI FNQWLTEAEQ FLRKTQIPEN WEHAKYKWYL KATMQDLEQR RPQLEELITA AQNLKNKTSN QEARTIITDR IERIQNQWDE VQEHLQNRRQ QLNEMLKDST QWLEAKEEAE QVLGQARAKL ESWKEGPYTV DAIQKKITET KQLAKDLRQW QTNVDVANDL ALKLLRDYSA DDTRKVHMIT ENINASWRSI HKRVSEREAA LEETHRLLQQ FPLDLEKFLA WLTEAETTAN VLQDATRKER LLEDSKGVKE LMKQWQDLQG EIEAHTDVYH NLDENSQKIL RSLEGSDDAV LLQRRLDNMN FKWSELRKKS LNIRSHLEAS SDQWKRLHLS LQELLVWLQL KDDELSRQAP IGGDFPAVQK QNDVHRAFKR ELKTKEPVIM STLETVRIFL TEQPLEGLEK LYQEPRELPP EERAQNVTRL LRKQAEEVNT EWEKLNLHSA DWQRKIDETL ERLQELQEAT DELDLKLRQA EVIKGSWQPV GDLLIDSLQD HLEKVKALRG EIAPLKENVS HVNDLARQLT TLGIQLSPYN LSTLEDLNTR WKLLQVAVED RVRQLHE |
-Experimental information
| Beam | Instrument name: SOLEIL SWING  / City: Saint-Aubin / 国: France / City: Saint-Aubin / 国: France  / Type of source: X-ray synchrotron / Wavelength: 0.1033 Å / Dist. spec. to detc.: 1.8 mm / Type of source: X-ray synchrotron / Wavelength: 0.1033 Å / Dist. spec. to detc.: 1.8 mm | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Detector | Name: AVIEX PCCD170170 / Type: CCD | ||||||||||||||||||
| Scan |
| ||||||||||||||||||
| Distance distribution function P(R) |
| ||||||||||||||||||
| Result | Comments: SEC-SAXS was performed at 15°C using the following parameters: Column: BioSEC5-500Å (4.6 mm id * 300 mm); Flow rate: 0.3 mL/min; Sample injection concentration: 5 mg/mL; Injection volume: ...Comments: SEC-SAXS was performed at 15°C using the following parameters: Column: BioSEC5-500Å (4.6 mm id * 300 mm); Flow rate: 0.3 mL/min; Sample injection concentration: 5 mg/mL; Injection volume: 70μL. The data were collected through the SEC peak of the protein as a series of several x 0.75 second exposures. The experimental molecular weight was determined from the volume of correlation, Vc.
|
 Movie
Movie Controller
Controller







