[English] 日本語
 Yorodumi
Yorodumi- SASDDM9: lipoprotein lipase–GPIHBP1 complex (Lipoprotein lipase, hLPL + Gl... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: SASBDB / ID: SASDDM9 |
|---|---|
 Sample Sample | lipoprotein lipase–GPIHBP1 complex
|
| Function / homology |  Function and homology information Function and homology informationlipoprotein lipase / low-density lipoprotein particle mediated signaling / lipoprotein lipase activity / chylomicron remodeling / positive regulation of cholesterol storage / phospholipase A1 / glycerophospholipase activity / glycerophospholipid phospholipase A1 activity / Assembly of active LPL and LIPC lipase complexes / triglyceride catabolic process ...lipoprotein lipase / low-density lipoprotein particle mediated signaling / lipoprotein lipase activity / chylomicron remodeling / positive regulation of cholesterol storage / phospholipase A1 / glycerophospholipase activity / glycerophospholipid phospholipase A1 activity / Assembly of active LPL and LIPC lipase complexes / triglyceride catabolic process / very-low-density lipoprotein particle remodeling / Chylomicron remodeling / very-low-density lipoprotein particle clearance / chylomicron / positive regulation of lipid storage / high-density lipoprotein particle remodeling / cellular response to nutrient / triacylglycerol lipase activity / very-low-density lipoprotein particle / heparan sulfate proteoglycan binding / positive regulation of macrophage derived foam cell differentiation / cellular response to fatty acid / positive regulation of chemokine (C-X-C motif) ligand 2 production / triglyceride homeostasis / triglyceride metabolic process / lipoprotein particle binding / apolipoprotein binding / positive regulation of fat cell differentiation / response to glucose / catalytic complex / retinoid metabolic process / Retinoid metabolism and transport / positive regulation of chemokine production / protein-membrane adaptor activity / positive regulation of adipose tissue development / phospholipid metabolic process / cholesterol homeostasis / positive regulation of interleukin-1 beta production / response to bacterium / fatty acid metabolic process / Transcriptional regulation of white adipocyte differentiation / positive regulation of interleukin-6 production / positive regulation of tumor necrosis factor production / positive regulation of inflammatory response / fatty acid biosynthetic process / heparin binding / MLL4 and MLL3 complexes regulate expression of PPARG target genes in adipogenesis and hepatic steatosis / signaling receptor binding / calcium ion binding / cell surface / protein homodimerization activity / extracellular space / extracellular region / plasma membrane Similarity search - Function |
| Biological species |  Homo sapiens (human) Homo sapiens (human) |
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2019 Journal: Proc Natl Acad Sci U S A / Year: 2019Title: Structure of the lipoprotein lipase-GPIHBP1 complex that mediates plasma triglyceride hydrolysis. Authors: Gabriel Birrane / Anne P Beigneux / Brian Dwyer / Bettina Strack-Logue / Kristian Kølby Kristensen / Omar L Francone / Loren G Fong / Haydyn D T Mertens / Clark Q Pan / Michael Ploug / ...Authors: Gabriel Birrane / Anne P Beigneux / Brian Dwyer / Bettina Strack-Logue / Kristian Kølby Kristensen / Omar L Francone / Loren G Fong / Haydyn D T Mertens / Clark Q Pan / Michael Ploug / Stephen G Young / Muthuraman Meiyappan /    Abstract: Lipoprotein lipase (LPL) is responsible for the intravascular processing of triglyceride-rich lipoproteins. The LPL within capillaries is bound to GPIHBP1, an endothelial cell protein with a three- ...Lipoprotein lipase (LPL) is responsible for the intravascular processing of triglyceride-rich lipoproteins. The LPL within capillaries is bound to GPIHBP1, an endothelial cell protein with a three-fingered LU domain and an N-terminal intrinsically disordered acidic domain. Loss-of-function mutations in or cause severe hypertriglyceridemia (chylomicronemia), but structures for LPL and GPIHBP1 have remained elusive. Inspired by our recent discovery that GPIHBP1's acidic domain preserves LPL structure and activity, we crystallized an LPL-GPIHBP1 complex and solved its structure. GPIHBP1's LU domain binds to LPL's C-terminal domain, largely by hydrophobic interactions. Analysis of electrostatic surfaces revealed that LPL contains a large basic patch spanning its N- and C-terminal domains. GPIHBP1's acidic domain was not defined in the electron density map but was positioned to interact with LPL's large basic patch, providing a likely explanation for how GPIHBP1 stabilizes LPL. The LPL-GPIHBP1 structure provides insights into mutations causing chylomicronemia. |
 Contact author Contact author |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
-Data source
| SASBDB page |  SASDDM9 SASDDM9 |
|---|
-Related structure data
- External links
External links
| Related items in Molecule of the Month |
|---|
-Models
| Model #2159 | 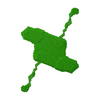 Type: dummy / Software: (2.8.1) / Radius of dummy atoms: 2.20 A / Symmetry: P2 / Chi-square value: 1.067 / P-value: 0.382446  Search similar-shape structures of this assembly by Omokage search (details) Search similar-shape structures of this assembly by Omokage search (details) |
|---|
- Sample
Sample
 Sample Sample | Name: lipoprotein lipase–GPIHBP1 complex / Specimen concentration: 0.72 mg/ml / Entity id: 1184 / 1185 |
|---|---|
| Buffer | Name: 10 mM BisTris, 150 mM NaCl, 4 mM CaCl2, 10% glycerol / pH: 7.4 |
| Entity #1184 | Name: hLPL / Type: protein / Description: Lipoprotein lipase / Formula weight: 50.323 / Num. of mol.: 2 / Source: Homo sapiens / References: UniProt: P06858 Sequence: DQRRDFIDIE SKFALRTPED TAEDTCHLIP GVAESVATCH FNHSSKTFMV IHGWTVTGMY ESWVPKLVAA LYKREPDSNV IVVDWLSRAQ EHYPVSAGYT KLVGQDVARF INWMEEEFNY PLDNVHLLGY SLGAHAAGIA GSLTNKKVNR ITGLDPAGPN FEYAEAPSRL ...Sequence: DQRRDFIDIE SKFALRTPED TAEDTCHLIP GVAESVATCH FNHSSKTFMV IHGWTVTGMY ESWVPKLVAA LYKREPDSNV IVVDWLSRAQ EHYPVSAGYT KLVGQDVARF INWMEEEFNY PLDNVHLLGY SLGAHAAGIA GSLTNKKVNR ITGLDPAGPN FEYAEAPSRL SPDDADFVDV LHTFTRGSPG RSIGIQKPVG HVDIYPNGGT FQPGCNIGEA IRVIAERGLG DVDQLVKCSH ERSIHLFIDS LLNEENPSKA YRCSSKEAFE KGLCLSCRKN RCNNLGYEIN KVRAKRSSKM YLKTRSQMPY KVFHYQVKIH FSGTESETHT NQAFEISLYG TVAESENIPF TLPEVSTNKT YSFLIYTEVD IGELLMLKLK WKSDSYFSWS DWWSSPGFAI QKIRVKAGET QKKVIFCSRE KVSHLQKGKA PAVFVKCHDK SLNKKSG |
| Entity #1185 | Name: GPIHBP1 / Type: protein Description: Glycosylphosphatidylinositol-anchored high density lipoprotein-binding protein 1 Formula weight: 14.713 / Num. of mol.: 2 / Source: Homo sapiens Sequence: QTQQEEEEED EDHGPDDYDE EDEDEVEEEE TNRLPGGRSR VLLRCYTCKS LPRDERCNLT QNCSHGQTCT TLIAHGNTES GLLTTHSTWC TDSCQPITKT VEGTQVTMTC CQSSLCNVPP WQSSRVQDPT G |
-Experimental information
| Beam | Instrument name: PETRA III EMBL P12 / City: Hamburg / 国: Germany  / Type of source: X-ray synchrotron / Wavelength: 0.124 Å / Dist. spec. to detc.: 3.1 mm / Type of source: X-ray synchrotron / Wavelength: 0.124 Å / Dist. spec. to detc.: 3.1 mm | ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Detector | Name: Pilatus 2M | ||||||||||||||||||||||||||||||||||||||||||
| Scan |
| ||||||||||||||||||||||||||||||||||||||||||
| Distance distribution function P(R) |
| ||||||||||||||||||||||||||||||||||||||||||
| Result |
|
 Movie
Movie Controller
Controller






