[English] 日本語
 Yorodumi
Yorodumi- PDB-8fwq: Structure of kainate receptor GluK2 in complex with the positive ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8fwq | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of kainate receptor GluK2 in complex with the positive allosteric modulator BPAM344 | |||||||||
 Components Components | Glutamate receptor ionotropic, kainate 2 | |||||||||
 Keywords Keywords | MEMBRANE PROTEIN / ion channel / iGluR / Kainate receptor / positive allosteric modulator | |||||||||
| Function / homology |  Function and homology information Function and homology informationmossy fiber rosette / detection of cold stimulus involved in thermoception / Activation of Na-permeable kainate receptors / Activation of Ca-permeable Kainate Receptor / kainate selective glutamate receptor complex / regulation of short-term neuronal synaptic plasticity / glutamate receptor activity / ubiquitin conjugating enzyme binding / negative regulation of synaptic transmission, glutamatergic / regulation of JNK cascade ...mossy fiber rosette / detection of cold stimulus involved in thermoception / Activation of Na-permeable kainate receptors / Activation of Ca-permeable Kainate Receptor / kainate selective glutamate receptor complex / regulation of short-term neuronal synaptic plasticity / glutamate receptor activity / ubiquitin conjugating enzyme binding / negative regulation of synaptic transmission, glutamatergic / regulation of JNK cascade / inhibitory postsynaptic potential / receptor clustering / kainate selective glutamate receptor activity / modulation of excitatory postsynaptic potential / extracellularly glutamate-gated ion channel activity / ionotropic glutamate receptor complex / positive regulation of synaptic transmission / behavioral fear response / neuronal action potential / glutamate-gated receptor activity / glutamate-gated calcium ion channel activity / ligand-gated monoatomic ion channel activity involved in regulation of presynaptic membrane potential / presynaptic modulation of chemical synaptic transmission / dendrite cytoplasm / hippocampal mossy fiber to CA3 synapse / SNARE binding / PDZ domain binding / regulation of membrane potential / excitatory postsynaptic potential / transmitter-gated monoatomic ion channel activity involved in regulation of postsynaptic membrane potential / synaptic transmission, glutamatergic / regulation of long-term neuronal synaptic plasticity / postsynaptic density membrane / modulation of chemical synaptic transmission / intracellular calcium ion homeostasis / terminal bouton / positive regulation of neuron apoptotic process / presynaptic membrane / neuron apoptotic process / scaffold protein binding / perikaryon / chemical synaptic transmission / negative regulation of neuron apoptotic process / postsynaptic membrane / postsynaptic density / axon / neuronal cell body / ubiquitin protein ligase binding / dendrite / synapse / glutamatergic synapse / identical protein binding / membrane / plasma membrane Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 3.96 Å | |||||||||
 Authors Authors | Gangwar, S.P. / Yen, L.Y. / Yelshanskaya, M.V. / Sobolevsky, A.I. | |||||||||
| Funding support |  United States, 2items United States, 2items
| |||||||||
 Citation Citation |  Journal: Cell Rep / Year: 2023 Journal: Cell Rep / Year: 2023Title: Positive and negative allosteric modulation of GluK2 kainate receptors by BPAM344 and antiepileptic perampanel. Authors: Shanti Pal Gangwar / Laura Y Yen / Maria V Yelshanskaya / Alexander I Sobolevsky /  Abstract: Kainate receptors (KARs) are a subtype of ionotropic glutamate receptors that control synaptic transmission in the central nervous system and are implicated in neurological, psychiatric, and ...Kainate receptors (KARs) are a subtype of ionotropic glutamate receptors that control synaptic transmission in the central nervous system and are implicated in neurological, psychiatric, and neurodevelopmental disorders. Understanding the regulation of KAR function by small molecules is essential for exploring these receptors as drug targets. Here, we present cryoelectron microscopy (cryo-EM) structures of KAR GluK2 in complex with the positive allosteric modulator BPAM344, competitive antagonist DNQX, and negative allosteric modulator, antiepileptic drug perampanel. Our structures show that two BPAM344 molecules bind per ligand-binding domain dimer interface. In the absence of an agonist or in the presence of DNQX, BPAM344 stabilizes GluK2 in the closed state. The closed state is also stabilized by perampanel, which binds to the ion channel extracellular collar sites located in two out of four GluK2 subunits. The molecular mechanisms of positive and negative allosteric modulation of KAR provide a guide for developing new therapeutic strategies. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8fwq.cif.gz 8fwq.cif.gz | 592.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8fwq.ent.gz pdb8fwq.ent.gz | 490.9 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  8fwq.json.gz 8fwq.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/fw/8fwq https://data.pdbj.org/pub/pdb/validation_reports/fw/8fwq ftp://data.pdbj.org/pub/pdb/validation_reports/fw/8fwq ftp://data.pdbj.org/pub/pdb/validation_reports/fw/8fwq | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  29515MC  8fwrC  8fwsC  8fwtC  8fwuC  8fwvC  8fwwC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Protein , 1 types, 4 molecules ABCD
| #1: Protein | Mass: 102509.977 Da / Num. of mol.: 4 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / References: UniProt: P42260 Homo sapiens (human) / References: UniProt: P42260 |
|---|
-Sugars , 5 types, 32 molecules 
| #2: Polysaccharide | 2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose Source method: isolated from a genetically manipulated source #3: Polysaccharide | Source method: isolated from a genetically manipulated source #4: Polysaccharide | beta-D-mannopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta- ...beta-D-mannopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose Source method: isolated from a genetically manipulated source #5: Polysaccharide | Source method: isolated from a genetically manipulated source #6: Sugar | ChemComp-NAG / |
|---|
-Non-polymers , 2 types, 5 molecules 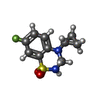


| #7: Chemical | ChemComp-2J9 / #8: Chemical | ChemComp-NA / | |
|---|
-Details
| Has ligand of interest | Y |
|---|---|
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: GluK2 / Type: COMPLEX Details: Map displaying amino-terminal, ligand binding, and transmembrane domain Entity ID: #1 / Source: RECOMBINANT | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular weight | Experimental value: NO | ||||||||||||||||||||
| Source (natural) | Organism:  | ||||||||||||||||||||
| Source (recombinant) | Organism:  Homo sapiens (human) Homo sapiens (human) | ||||||||||||||||||||
| Buffer solution | pH: 8 | ||||||||||||||||||||
| Buffer component |
| ||||||||||||||||||||
| Specimen | Conc.: 5 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES Details: Protein extracted and reconstituted in a detergent micelle | ||||||||||||||||||||
| Specimen support | Grid type: Quantifoil R1.2/1.3 | ||||||||||||||||||||
| Vitrification | Instrument: FEI VITROBOT MARK IV / Cryogen name: ETHANE / Humidity: 100 % / Chamber temperature: 277 K |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: SPOT SCAN FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: SPOT SCAN |
| Electron lens | Mode: BRIGHT FIELD / Nominal defocus max: 2000 nm / Nominal defocus min: 1000 nm |
| Image recording | Electron dose: 50 e/Å2 / Film or detector model: FEI FALCON IV (4k x 4k) |
- Processing
Processing
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3D reconstruction | Resolution: 3.96 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 28297 / Symmetry type: POINT | ||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller








 PDBj
PDBj




