+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8d2w | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Zebrafish MFSD2A isoform B in inward open ligand 2B conformation | ||||||||||||
 Components Components |
| ||||||||||||
 Keywords Keywords | LIPID TRANSPORT / omega-3 fatty acid / membrane protein / Fab | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationSynthesis of PC / lysophospholipid translocation / fatty acid transmembrane transporter activity / lysophospholipid:sodium symporter activity / lysophospholipid transport / lipid transport across blood-brain barrier / transcytosis / establishment of blood-brain barrier / symporter activity / carbohydrate transport ...Synthesis of PC / lysophospholipid translocation / fatty acid transmembrane transporter activity / lysophospholipid:sodium symporter activity / lysophospholipid transport / lipid transport across blood-brain barrier / transcytosis / establishment of blood-brain barrier / symporter activity / carbohydrate transport / fatty acid transport / transmembrane transport / endoplasmic reticulum membrane / plasma membrane Similarity search - Function | ||||||||||||
| Biological species |   | ||||||||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 3.4 Å | ||||||||||||
 Authors Authors | Nguyen, C. / Lei, H.T. / Lai, L.T.F. / Gallentino, M.J. / Mu, X. / Matthies, D. / Gonen, T. | ||||||||||||
| Funding support |  United States, 3items United States, 3items
| ||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: Lipid flipping in the omega-3 fatty-acid transporter. Authors: Chi Nguyen / Hsiang-Ting Lei / Louis Tung Faat Lai / Marc J Gallenito / Xuelang Mu / Doreen Matthies / Tamir Gonen /  Abstract: Mfsd2a is the transporter for docosahexaenoic acid (DHA), an omega-3 fatty acid, across the blood brain barrier (BBB). Defects in Mfsd2a are linked to ailments from behavioral and motor dysfunctions ...Mfsd2a is the transporter for docosahexaenoic acid (DHA), an omega-3 fatty acid, across the blood brain barrier (BBB). Defects in Mfsd2a are linked to ailments from behavioral and motor dysfunctions to microcephaly. Mfsd2a transports long-chain unsaturated fatty-acids, including DHA and α-linolenic acid (ALA), that are attached to the zwitterionic lysophosphatidylcholine (LPC) headgroup. Even with the recently determined structures of Mfsd2a, the molecular details of how this transporter performs the energetically unfavorable task of translocating and flipping lysolipids across the lipid bilayer remains unclear. Here, we report five single-particle cryo-EM structures of Danio rerio Mfsd2a (drMfsd2a): in the inward-open conformation in the ligand-free state and displaying lipid-like densities modeled as ALA-LPC at four distinct positions. These Mfsd2a snapshots detail the flipping mechanism for lipid-LPC from outer to inner membrane leaflet and release for membrane integration on the cytoplasmic side. These results also map Mfsd2a mutants that disrupt lipid-LPC transport and are associated with disease. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8d2w.cif.gz 8d2w.cif.gz | 186.7 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8d2w.ent.gz pdb8d2w.ent.gz | 143.7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  8d2w.json.gz 8d2w.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/d2/8d2w https://data.pdbj.org/pub/pdb/validation_reports/d2/8d2w ftp://data.pdbj.org/pub/pdb/validation_reports/d2/8d2w ftp://data.pdbj.org/pub/pdb/validation_reports/d2/8d2w | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  27152MC  8d2sC  8d2tC  8d2uC  8d2vC  8d2xC  8d2r M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Antibody , 2 types, 2 molecules BC
| #2: Antibody | Mass: 20674.949 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   |
|---|---|
| #3: Antibody | Mass: 20568.018 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   |
-Protein / Sugars , 2 types, 16 molecules A

| #1: Protein | Mass: 56683.965 Da / Num. of mol.: 1 / Mutation: N214Q,N225Q,N509Q Source method: isolated from a genetically manipulated source Source: (gene. exp.)   |
|---|---|
| #5: Sugar | ChemComp-LMT / |
-Non-polymers , 2 types, 6 molecules 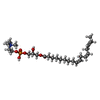


| #4: Chemical | ChemComp-ZGS / [( |
|---|---|
| #6: Water | ChemComp-HOH / |
-Details
| Has ligand of interest | Y |
|---|---|
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Single-particle cryo-EM map of MFSD2A isoform B from zebrafish with a Fab in the inward open ligand 2B conformation at an average resolution of 3.4 A, filtered to local resolution Type: COMPLEX / Entity ID: #1-#3 / Source: MULTIPLE SOURCES | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular weight | Value: 0.056 MDa / Experimental value: NO | ||||||||||||||||
| Source (natural) |
| ||||||||||||||||
| Source (recombinant) |
| ||||||||||||||||
| Buffer solution | pH: 8 / Details: 50 mM HEPES (pH 8), 150 mM NaCl and 0.02% DDM | ||||||||||||||||
| Buffer component |
| ||||||||||||||||
| Specimen | Conc.: 3 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES Details: Purified 15A9 FAB fragment was incubated overnight at 4 C with MFSD2A at a 5:1 molar ratio. DrMFSD2A-15A9 complex was injected into the size exclusion column Superdex200 to separate the ...Details: Purified 15A9 FAB fragment was incubated overnight at 4 C with MFSD2A at a 5:1 molar ratio. DrMFSD2A-15A9 complex was injected into the size exclusion column Superdex200 to separate the unbound FAB. The size exclusion column step was also used to exchange the buffer of the MFSD2A-FAB complex into 50 mM HEPES, 150 mM NaCl and 0.02% DDM. SDS-PAGE was used to pool peak fractions containing both MFSD2A and FAB, indicating complex formation. The pooled fractions containing MFSD2A-FAB were concentrated to 3 mg/ml using Amicon spin concentrator with a 30 kDa cutoff. | ||||||||||||||||
| Specimen support | Grid material: COPPER / Grid mesh size: 400 divisions/in. / Grid type: Quantifoil R1.2/1.3 | ||||||||||||||||
| Vitrification | Instrument: LEICA EM GP / Cryogen name: ETHANE / Humidity: 86 % / Chamber temperature: 277 K Details: 400-mesh 1.2/1.3 Cu grids (Quantifoil) were made hydrophilic by glow discharging for two times 60 seconds with a current of 15 mA in a PELCO easiGlow system. The cryo grids were produced ...Details: 400-mesh 1.2/1.3 Cu grids (Quantifoil) were made hydrophilic by glow discharging for two times 60 seconds with a current of 15 mA in a PELCO easiGlow system. The cryo grids were produced using a Leica EM GP (Leica). The chamber was kept at 4 C and 95% humidity (86-91% measured). 3 microliter sample at 3 mg/ml was applied to a glow-discharged holey grid, blotted for 6 s, and plunge frozen into liquid ethane and stored in liquid nitrogen. |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: TFS KRIOS Details: Cryo-EM grids were loaded into a 300 keV FEI Titan Krios cryo electron microscope (ThermoFisher Scientific, formerly FEI) at HHMI Janelia Reasearch Campus, Janelia Krios 1, equipped with a ...Details: Cryo-EM grids were loaded into a 300 keV FEI Titan Krios cryo electron microscope (ThermoFisher Scientific, formerly FEI) at HHMI Janelia Reasearch Campus, Janelia Krios 1, equipped with a Cs corrector, and Gatan energy filter and K3 camera (Gatan Inc.). Movies of 50 frames with 1 e-/A2 per frame (50 e-/A2 total dose) were automatically recorded at a nominal magnification of 81,000x, corresponding to a physical pixel size of 0.844 A/px (superresolution pixel size 0.422 A/px) in CDS mode at a dose rate of 9.5 e-/px/s (~7.5 e-/px/s on the camera through the sample) and a defocus range of -0.5 to -1.8 micrometer using SerialEM. In total, 2,653 and 8,443 movies were collected in two separate imaging sessions, with the first dataset on a 400-mesh copper grid and the second on a 300-mesh UltrAuFoil gold grid. |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Nominal magnification: 81000 X / Nominal defocus max: 1800 nm / Nominal defocus min: 500 nm / Cs: 0.01 mm / C2 aperture diameter: 100 µm / Alignment procedure: ZEMLIN TABLEAU |
| Specimen holder | Cryogen: NITROGEN / Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Image recording | Average exposure time: 3.75 sec. / Electron dose: 50 e/Å2 / Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Num. of grids imaged: 2 / Num. of real images: 11096 Details: Movies of 50 frames with 1 e-/A2 per frame (50 e-/A2 total dose) were automatically recorded at a nominal magnification of 81,000x, corresponding to a physical pixel size of 0.844 A/px ...Details: Movies of 50 frames with 1 e-/A2 per frame (50 e-/A2 total dose) were automatically recorded at a nominal magnification of 81,000x, corresponding to a physical pixel size of 0.844 A/px (superresolution pixel size 0.422 A/px) in CDS mode at a dose rate of 9.5 e-/px/s (~7.5 e-/px/s on the camera through the sample) and a defocus range of -0.5 to -1.8 mircometer using SerialEM. In total, 2,653 and 8,443 movies were collected in two separate imaging sessions, with the first dataset on a 400-mesh copper grid and the second on a 300-mesh UltrAuFoil gold grid. |
| EM imaging optics | Energyfilter name: GIF Bioquantum / Energyfilter slit width: 20 eV |
- Processing
Processing
| Software | Name: PHENIX / Version: 1.19.2_4158: / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EM software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||
| Image processing | Details: Movies of 50 frames with 1 e-/A2 per frame (50 e-/A2 total dose) were automatically recorded at a nominal magnification of 81,000x, corresponding to a physical pixel size of 0.844 A/px ...Details: Movies of 50 frames with 1 e-/A2 per frame (50 e-/A2 total dose) were automatically recorded at a nominal magnification of 81,000x, corresponding to a physical pixel size of 0.844 A/px (superresolution pixel size 0.422 A/px) in CDS mode at a dose rate of 9.5 e-/px/s (~7.5 e-/px/s on the camera through the sample) and a defocus range of -0.5 to -1.8 mircometer using SerialEM. In total, 2,653 and 8,443 movies were collected in two separate imaging sessions, with the first dataset on a 400-mesh copper grid and the second on a 300-mesh UltrAuFoil gold grid. | ||||||||||||||||||||||||||||||||||||||||||||||||||
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D reconstruction | Resolution: 3.4 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 76700 / Algorithm: BACK PROJECTION / Symmetry type: POINT | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic model building |
| ||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic model building |
| ||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller








 PDBj
PDBj







