+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8bob | ||||||
|---|---|---|---|---|---|---|---|
| Title | Structural basis for negative regulation of the maltose system | ||||||
 Components Components |
| ||||||
 Keywords Keywords | TRANSCRIPTION / STAND / maltose system / oligomerization | ||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of carbohydrate metabolic process / cysteine-S-conjugate beta-lyase activity / cysteine-S-conjugate beta-lyase / : / L-cysteine desulfhydrase activity / methionine biosynthetic process / pyridoxal phosphate binding / DNA-binding transcription factor binding / carbohydrate metabolic process / DNA-binding transcription factor activity ...positive regulation of carbohydrate metabolic process / cysteine-S-conjugate beta-lyase activity / cysteine-S-conjugate beta-lyase / : / L-cysteine desulfhydrase activity / methionine biosynthetic process / pyridoxal phosphate binding / DNA-binding transcription factor binding / carbohydrate metabolic process / DNA-binding transcription factor activity / positive regulation of DNA-templated transcription / protein homodimerization activity / DNA binding / ATP binding Similarity search - Function | ||||||
| Biological species |   | ||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 2.94 Å | ||||||
 Authors Authors | Chai, J. / Wu, Y. | ||||||
| Funding support |  Germany, 1items Germany, 1items
| ||||||
 Citation Citation |  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: Structural basis for negative regulation of the Escherichia coli maltose system. Authors: Yuang Wu / Yue Sun / Evelyne Richet / Zhifu Han / Jijie Chai /    Abstract: Proteins from the signal transduction ATPases with numerous domains (STAND) family are known to play an important role in innate immunity. However, it remains less well understood how they function ...Proteins from the signal transduction ATPases with numerous domains (STAND) family are known to play an important role in innate immunity. However, it remains less well understood how they function in transcriptional regulation. MalT is a bacterial STAND that controls the Escherichia coli maltose system. Inactive MalT is sequestered by different inhibitory proteins such as MalY. Here, we show that MalY interacts with one oligomerization interface of MalT to form a 2:2 complex. MalY represses MalT activity by blocking its oligomerization and strengthening ADP-mediated MalT autoinhibition. A loop region N-terminal to the nucleotide-binding domain (NBD) of MalT has a dual role in mediating MalT autoinhibition and activation. Structural comparison shows that ligand-binding induced oligomerization is required for stabilizing the C-terminal domains and conferring DNA-binding activity. Together, our study reveals the mechanism whereby a prokaryotic STAND is inhibited by a repressor protein and offers insights into signaling by STAND transcription activators. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8bob.cif.gz 8bob.cif.gz | 298 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8bob.ent.gz pdb8bob.ent.gz | 240.7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  8bob.json.gz 8bob.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  8bob_validation.pdf.gz 8bob_validation.pdf.gz | 1.2 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  8bob_full_validation.pdf.gz 8bob_full_validation.pdf.gz | 1.2 MB | Display | |
| Data in XML |  8bob_validation.xml.gz 8bob_validation.xml.gz | 54 KB | Display | |
| Data in CIF |  8bob_validation.cif.gz 8bob_validation.cif.gz | 79.8 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/bo/8bob https://data.pdbj.org/pub/pdb/validation_reports/bo/8bob ftp://data.pdbj.org/pub/pdb/validation_reports/bo/8bob ftp://data.pdbj.org/pub/pdb/validation_reports/bo/8bob | HTTPS FTP |
-Related structure data
| Related structure data | 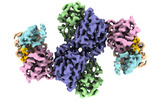 16140MC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
| #1: Protein | Mass: 43684.703 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   References: UniProt: P23256, cysteine-S-conjugate beta-lyase #2: Protein | Mass: 47318.680 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   #3: Chemical | #4: Chemical | Has ligand of interest | Y | |
|---|
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Binary complex of MalY and MalT / Type: CELL / Entity ID: #1-#2 / Source: NATURAL |
|---|---|
| Source (natural) | Organism:  |
| Buffer solution | pH: 8 |
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy imaging
Electron microscopy imaging
| Microscopy | Model: FEI TITAN |
|---|---|
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: SPOT SCAN FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: SPOT SCAN |
| Electron lens | Mode: BRIGHT FIELD / Nominal defocus max: 2000 nm / Nominal defocus min: 1200 nm |
| Image recording | Electron dose: 50 e/Å2 / Film or detector model: GATAN K2 SUMMIT (4k x 4k) |
- Processing
Processing
| Software | Name: PHENIX / Version: 1.15.2_3472: / Classification: refinement | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTF correction | Type: NONE | ||||||||||||||||||||||||
| 3D reconstruction | Resolution: 2.94 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 176969 / Symmetry type: POINT | ||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller




 PDBj
PDBj



