[English] 日本語
 Yorodumi
Yorodumi- PDB-7whz: SARS-CoV-2 spike protein in complex with three human neutralizing... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7whz | ||||||
|---|---|---|---|---|---|---|---|
| Title | SARS-CoV-2 spike protein in complex with three human neutralizing antibodies | ||||||
 Components Components |
| ||||||
 Keywords Keywords | VIRAL PROTEIN / SARS-CoV-2 / Omicron / Spike protein / Neutralizing antibody / Cryo-EM | ||||||
| Function / homology |  Function and homology information Function and homology informationMaturation of spike protein / viral translation / Translation of Structural Proteins / Virion Assembly and Release / host cell surface / host extracellular space / suppression by virus of host tetherin activity / Induction of Cell-Cell Fusion / structural constituent of virion / host cell endoplasmic reticulum-Golgi intermediate compartment membrane ...Maturation of spike protein / viral translation / Translation of Structural Proteins / Virion Assembly and Release / host cell surface / host extracellular space / suppression by virus of host tetherin activity / Induction of Cell-Cell Fusion / structural constituent of virion / host cell endoplasmic reticulum-Golgi intermediate compartment membrane / entry receptor-mediated virion attachment to host cell / receptor-mediated endocytosis of virus by host cell / Attachment and Entry / membrane fusion / positive regulation of viral entry into host cell / receptor-mediated virion attachment to host cell / receptor ligand activity / host cell surface receptor binding / fusion of virus membrane with host plasma membrane / fusion of virus membrane with host endosome membrane / viral envelope / virion attachment to host cell / SARS-CoV-2 activates/modulates innate and adaptive immune responses / host cell plasma membrane / virion membrane / membrane / identical protein binding / plasma membrane Similarity search - Function | ||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | ||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 3.42 Å | ||||||
 Authors Authors | Zheng, Q. / Li, S. / Sun, H. / Zheng, Z. / Wang, S. | ||||||
| Funding support | 1items
| ||||||
 Citation Citation |  Journal: Cell Rep / Year: 2022 Journal: Cell Rep / Year: 2022Title: Three SARS-CoV-2 antibodies provide broad and synergistic neutralization against variants of concern, including Omicron. Authors: Siling Wang / Hui Sun / Yali Zhang / Lunzhi Yuan / Yizhen Wang / Tianying Zhang / Shaojuan Wang / Jinlei Zhang / Hai Yu / Hualong Xiong / Zimin Tang / Liqin Liu / Yang Huang / Xiuting Chen / ...Authors: Siling Wang / Hui Sun / Yali Zhang / Lunzhi Yuan / Yizhen Wang / Tianying Zhang / Shaojuan Wang / Jinlei Zhang / Hai Yu / Hualong Xiong / Zimin Tang / Liqin Liu / Yang Huang / Xiuting Chen / Tingting Li / Dong Ying / Chang Liu / Zihao Chen / Quan Yuan / Jun Zhang / Tong Cheng / Shaowei Li / Yi Guan / Qingbing Zheng / Zizheng Zheng / Ningshao Xia /  Abstract: The rapidly spreading Omicron variant is highly resistant to vaccines, convalescent sera, and neutralizing antibodies (nAbs), highlighting the urgent need for potent therapeutic nAbs. Here, a panel ...The rapidly spreading Omicron variant is highly resistant to vaccines, convalescent sera, and neutralizing antibodies (nAbs), highlighting the urgent need for potent therapeutic nAbs. Here, a panel of human nAbs from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) convalescent patients show diverse neutralization against Omicron, of which XMA01 and XMA04 maintain nanomolar affinities and excellent neutralization (half maximal inhibitory concentration [IC50]: ∼20 ng/mL). nAb XMA09 shows weak but unattenuated neutralization against all variants of concern (VOCs) as well as SARS-CoV. Structural analysis reveals that the above three antibodies could synergistically bind to the receptor-binding domains (RBDs) of both wild-type and Omicron spikes and defines the critical determinants for nAb-mediated broad neutralizations. Three nAbs confer synergistic neutralization against Omicron, resulting from the inter-antibody interaction between XMA04 and XMA01(or XMA09). Furthermore, the XMA01/XMA04 cocktail provides synergistic protection against Beta and Omicron variant infections in hamsters. In summary, our results provide insights for the rational design of antibody cocktail therapeutics or universal vaccines against Omicron. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7whz.cif.gz 7whz.cif.gz | 157.5 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7whz.ent.gz pdb7whz.ent.gz | 126.8 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7whz.json.gz 7whz.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  7whz_validation.pdf.gz 7whz_validation.pdf.gz | 836.3 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  7whz_full_validation.pdf.gz 7whz_full_validation.pdf.gz | 843.6 KB | Display | |
| Data in XML |  7whz_validation.xml.gz 7whz_validation.xml.gz | 31.2 KB | Display | |
| Data in CIF |  7whz_validation.cif.gz 7whz_validation.cif.gz | 46.1 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/wh/7whz https://data.pdbj.org/pub/pdb/validation_reports/wh/7whz ftp://data.pdbj.org/pub/pdb/validation_reports/wh/7whz ftp://data.pdbj.org/pub/pdb/validation_reports/wh/7whz | HTTPS FTP |
-Related structure data
| Related structure data | 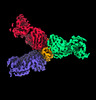 32516MC  7wi0C M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Antibody , 6 types, 6 molecules BCDEHL
| #2: Antibody | Mass: 13517.036 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Production host: Homo sapiens (human) / Production host:  Homo sapiens (human) Homo sapiens (human) |
|---|---|
| #3: Antibody | Mass: 13198.615 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Production host: Homo sapiens (human) / Production host:  Homo sapiens (human) Homo sapiens (human) |
| #4: Antibody | Mass: 11616.873 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Production host: Homo sapiens (human) / Production host:  Homo sapiens (human) / References: UniProt: A0A5C2G4I7 Homo sapiens (human) / References: UniProt: A0A5C2G4I7 |
| #5: Antibody | Mass: 11781.073 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Production host: Homo sapiens (human) / Production host:  Homo sapiens (human) / References: UniProt: A0A5C2GTV1 Homo sapiens (human) / References: UniProt: A0A5C2GTV1 |
| #6: Antibody | Mass: 13272.664 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Production host: Homo sapiens (human) / Production host:  Homo sapiens (human) Homo sapiens (human) |
| #7: Antibody | Mass: 11374.391 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Production host: Homo sapiens (human) / Production host:  Homo sapiens (human) / References: UniProt: A0A5C2GVU3 Homo sapiens (human) / References: UniProt: A0A5C2GVU3 |
-Protein / Sugars , 2 types, 2 molecules A

| #1: Protein | Mass: 21873.496 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Gene: S, 2 / Production host:  Homo sapiens (human) / References: UniProt: P0DTC2 Homo sapiens (human) / References: UniProt: P0DTC2 |
|---|---|
| #8: Sugar | ChemComp-NAG / |
-Details
| Has ligand of interest | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: SARS-CoV-2 spike protein in complex with three human neutralizing antibodies Type: COMPLEX / Entity ID: #1-#7 / Source: RECOMBINANT |
|---|---|
| Source (natural) | Organism:  |
| Source (recombinant) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Buffer solution | pH: 7.4 |
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Tecnai F30 / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TECNAI F30 |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Nominal defocus max: 2000 nm / Nominal defocus min: 800 nm |
| Image recording | Electron dose: 60 e/Å2 / Film or detector model: GATAN K3 (6k x 4k) |
- Processing
Processing
| Software | Name: PHENIX / Version: 1.14_3259: / Classification: refinement |
|---|---|
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION |
| 3D reconstruction | Resolution: 3.42 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 721486 / Symmetry type: POINT |
 Movie
Movie Controller
Controller




 PDBj
PDBj






