+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7sc8 | ||||||
|---|---|---|---|---|---|---|---|
| Title | Synechocystis PCC 6803 Phycobilisome rod from PBS sample | ||||||
 Components Components |
| ||||||
 Keywords Keywords | PHOTOSYNTHESIS / Complex / light harvesting / pigment | ||||||
| Function / homology |  Function and homology information Function and homology informationphycobilisome / plasma membrane-derived thylakoid membrane / photosynthesis Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 2.1 Å | ||||||
 Authors Authors | Sauer, P.V. / Sutter, M. / Dominguez-Martin, M.A. / Kirst, H. / Kerfeld, C.A. | ||||||
| Funding support | European Union, 1items
| ||||||
 Citation Citation |  Journal: Nature / Year: 2022 Journal: Nature / Year: 2022Title: Structures of a phycobilisome in light-harvesting and photoprotected states. Authors: María Agustina Domínguez-Martín / Paul V Sauer / Henning Kirst / Markus Sutter / David Bína / Basil J Greber / Eva Nogales / Tomáš Polívka / Cheryl A Kerfeld /    Abstract: Phycobilisome (PBS) structures are elaborate antennae in cyanobacteria and red algae. These large protein complexes capture incident sunlight and transfer the energy through a network of embedded ...Phycobilisome (PBS) structures are elaborate antennae in cyanobacteria and red algae. These large protein complexes capture incident sunlight and transfer the energy through a network of embedded pigment molecules called bilins to the photosynthetic reaction centres. However, light harvesting must also be balanced against the risks of photodamage. A known mode of photoprotection is mediated by orange carotenoid protein (OCP), which binds to PBS when light intensities are high to mediate photoprotective, non-photochemical quenching. Here we use cryogenic electron microscopy to solve four structures of the 6.2 MDa PBS, with and without OCP bound, from the model cyanobacterium Synechocystis sp. PCC 6803. The structures contain a previously undescribed linker protein that binds to the membrane-facing side of PBS. For the unquenched PBS, the structures also reveal three different conformational states of the antenna, two previously unknown. The conformational states result from positional switching of two of the rods and may constitute a new mode of regulation of light harvesting. Only one of the three PBS conformations can bind to OCP, which suggests that not every PBS is equally susceptible to non-photochemical quenching. In the OCP-PBS complex, quenching is achieved through the binding of four 34 kDa OCPs organized as two dimers. The complex reveals the structure of the active form of OCP, in which an approximately 60 Å displacement of its regulatory carboxy terminal domain occurs. Finally, by combining our structure with spectroscopic properties, we elucidate energy transfer pathways within PBS in both the quenched and light-harvesting states. Collectively, our results provide detailed insights into the biophysical underpinnings of the control of cyanobacterial light harvesting. The data also have implications for bioengineering PBS regulation in natural and artificial light-harvesting systems. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7sc8.cif.gz 7sc8.cif.gz | 1.2 MB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7sc8.ent.gz pdb7sc8.ent.gz | Display |  PDB format PDB format | |
| PDBx/mmJSON format |  7sc8.json.gz 7sc8.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  7sc8_validation.pdf.gz 7sc8_validation.pdf.gz | 4.2 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  7sc8_full_validation.pdf.gz 7sc8_full_validation.pdf.gz | 4.3 MB | Display | |
| Data in XML |  7sc8_validation.xml.gz 7sc8_validation.xml.gz | 190.1 KB | Display | |
| Data in CIF |  7sc8_validation.cif.gz 7sc8_validation.cif.gz | 286.9 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/sc/7sc8 https://data.pdbj.org/pub/pdb/validation_reports/sc/7sc8 ftp://data.pdbj.org/pub/pdb/validation_reports/sc/7sc8 ftp://data.pdbj.org/pub/pdb/validation_reports/sc/7sc8 | HTTPS FTP |
-Related structure data
| Related structure data |  25029MC  7sc7C  7sc9C  7scaC  7scbC  7sccC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
| Experimental dataset #1 | Data reference:  10.6019/EMPIAR-11133 / Data set type: EMPIAR / Details: EMPIAR-11133 10.6019/EMPIAR-11133 / Data set type: EMPIAR / Details: EMPIAR-11133 |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-C-phycocyanin ... , 2 types, 36 molecules AAACAEAGAIAKAMAOAQASAUAWAYBABCBEBGBIABADAFAHAJALANAPARATAVAX...
| #1: Protein | Mass: 17602.529 Da / Num. of mol.: 18 / Source method: isolated from a natural source Source: (natural)  Strain: PCC 6803 / Kazusa / References: UniProt: Q54715 #2: Protein | Mass: 18142.426 Da / Num. of mol.: 18 / Source method: isolated from a natural source Source: (natural)  Strain: PCC 6803 / Kazusa / References: UniProt: Q54714 |
|---|
-Phycobilisome ... , 4 types, 4 molecules BKBLBMBN
| #3: Protein | Mass: 28938.758 Da / Num. of mol.: 1 / Source method: isolated from a natural source Source: (natural)  Strain: PCC 6803 / Kazusa / References: UniProt: P73093 |
|---|---|
| #4: Protein | Mass: 32558.607 Da / Num. of mol.: 1 / Source method: isolated from a natural source Source: (natural)  Strain: PCC 6803 / Kazusa / References: UniProt: P73203 |
| #5: Protein | Mass: 30836.346 Da / Num. of mol.: 1 / Source method: isolated from a natural source Source: (natural)  Strain: PCC 6803 / Kazusa / References: UniProt: P73204 |
| #6: Protein | Mass: 9333.342 Da / Num. of mol.: 1 / Source method: isolated from a natural source Source: (natural)  Strain: PCC 6803 / Kazusa / References: UniProt: P73202 |
-Non-polymers , 2 types, 2726 molecules 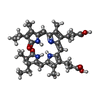


| #7: Chemical | ChemComp-CYC / #8: Water | ChemComp-HOH / | |
|---|
-Details
| Has ligand of interest | Y |
|---|---|
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Phycobilisome from Synechocystis PCC 6803 / Type: COMPLEX / Entity ID: #1-#6 / Source: NATURAL |
|---|---|
| Molecular weight | Experimental value: NO |
| Source (natural) | Organism:  |
| Buffer solution | pH: 7.5 |
| Specimen | Conc.: 3.8 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES |
| Specimen support | Grid material: COPPER / Grid type: Quantifoil R2/1 |
| Vitrification | Instrument: FEI VITROBOT MARK IV / Cryogen name: ETHANE-PROPANE / Details: Manual blotting |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: TFS KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: OTHER FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: OTHER |
| Electron lens | Mode: BRIGHT FIELD / Nominal defocus max: 1800 nm / Nominal defocus min: 600 nm |
| Specimen holder | Cryogen: NITROGEN |
| Image recording | Electron dose: 50 e/Å2 / Film or detector model: GATAN K3 (6k x 4k) |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EM software |
| ||||||||||||||||||||||||
| Image processing | Details: Streptavidin lattice was subtracted after movie alignment. | ||||||||||||||||||||||||
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||
| 3D reconstruction | Resolution: 2.1 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 2102860 / Symmetry type: POINT | ||||||||||||||||||||||||
| Atomic model building | Protocol: BACKBONE TRACE / Space: REAL | ||||||||||||||||||||||||
| Refinement | Cross valid method: NONE Stereochemistry target values: GeoStd + Monomer Library + CDL v1.2 | ||||||||||||||||||||||||
| Displacement parameters | Biso mean: 16.11 Å2 | ||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj
