+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7qpa | ||||||
|---|---|---|---|---|---|---|---|
| Title | Outward-facing auxin bound form of auxin transporter PIN8 | ||||||
 Components Components | Auxin efflux carrier component 8 | ||||||
 Keywords Keywords | MEMBRANE PROTEIN / Auxin transport / AEC family / BART superfamily | ||||||
| Function / homology |  Function and homology information Function and homology informationauxin export across the plasma membrane / auxin efflux transmembrane transporter activity / pollen development / auxin-activated signaling pathway / cell periphery / endoplasmic reticulum membrane / endoplasmic reticulum / protein homodimerization activity / identical protein binding / plasma membrane Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 3.18 Å | ||||||
 Authors Authors | Ung, K.L. / Winkler, M.B.L. / Dedic, E. / Stokes, D.L. / Pedersen, B.P. | ||||||
| Funding support | European Union, 1items
| ||||||
 Citation Citation |  Journal: Nature / Year: 2022 Journal: Nature / Year: 2022Title: Structures and mechanism of the plant PIN-FORMED auxin transporter. Authors: Kien Lam Ung / Mikael Winkler / Lukas Schulz / Martina Kolb / Dorina P Janacek / Emil Dedic / David L Stokes / Ulrich Z Hammes / Bjørn Panyella Pedersen /    Abstract: Auxins are hormones that have central roles and control nearly all aspects of growth and development in plants. The proteins in the PIN-FORMED (PIN) family (also known as the auxin efflux carrier ...Auxins are hormones that have central roles and control nearly all aspects of growth and development in plants. The proteins in the PIN-FORMED (PIN) family (also known as the auxin efflux carrier family) are key participants in this process and control auxin export from the cytosol to the extracellular space. Owing to a lack of structural and biochemical data, the molecular mechanism of PIN-mediated auxin transport is not understood. Here we present biophysical analysis together with three structures of Arabidopsis thaliana PIN8: two outward-facing conformations with and without auxin, and one inward-facing conformation bound to the herbicide naphthylphthalamic acid. The structure forms a homodimer, with each monomer divided into a transport and scaffold domain with a clearly defined auxin binding site. Next to the binding site, a proline-proline crossover is a pivot point for structural changes associated with transport, which we show to be independent of proton and ion gradients and probably driven by the negative charge of the auxin. The structures and biochemical data reveal an elevator-type transport mechanism reminiscent of bile acid/sodium symporters, bicarbonate/sodium symporters and sodium/proton antiporters. Our results provide a comprehensive molecular model for auxin recognition and transport by PINs, link and expand on a well-known conceptual framework for transport, and explain a central mechanism of polar auxin transport, a core feature of plant physiology, growth and development. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7qpa.cif.gz 7qpa.cif.gz | 234.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7qpa.ent.gz pdb7qpa.ent.gz | 191.9 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7qpa.json.gz 7qpa.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/qp/7qpa https://data.pdbj.org/pub/pdb/validation_reports/qp/7qpa ftp://data.pdbj.org/pub/pdb/validation_reports/qp/7qpa ftp://data.pdbj.org/pub/pdb/validation_reports/qp/7qpa | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  14116MC  7qp9C  7qpcC C: citing same article ( M: map data used to model this data |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
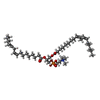
| #1: Protein | Mass: 41541.039 Da / Num. of mol.: 2 Mutation: First two residues MG are cloning tags. Uniprot sequence aligns from Ile2. Note MG is added as residue 0 and 1, to maintain correct numbering compared to uniprot. Source method: isolated from a genetically manipulated source Source: (gene. exp.)   #2: Chemical | #3: Chemical | #4: Water | ChemComp-HOH / | Has ligand of interest | Y | Has protein modification | N | |
|---|
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: IAA bound form of PIN8 / Type: COMPLEX / Details: dimer form of PIN8 / Entity ID: #1 / Source: RECOMBINANT | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular weight | Value: 0.081 MDa / Experimental value: NO | ||||||||||||||||||||
| Source (natural) | Organism:  | ||||||||||||||||||||
| Source (recombinant) | Organism:  | ||||||||||||||||||||
| Buffer solution | pH: 7.5 | ||||||||||||||||||||
| Buffer component |
| ||||||||||||||||||||
| Specimen | Conc.: 10 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES | ||||||||||||||||||||
| Specimen support | Details: The grid was glow-discharge at 15 mA / Grid material: COPPER / Grid mesh size: 300 divisions/in. / Grid type: C-flat-1.2/1.3 | ||||||||||||||||||||
| Vitrification | Instrument: FEI VITROBOT MARK IV / Cryogen name: ETHANE / Humidity: 100 % / Chamber temperature: 277.15 K Details: Wait 4 seconds after sample loading, Blotting time 4 seconds with blotting force of -1 before plunging |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Nominal magnification: 130000 X / Nominal defocus max: 2500 nm / Nominal defocus min: 500 nm / Cs: 2.7 mm / Alignment procedure: COMA FREE |
| Specimen holder | Cryogen: NITROGEN / Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Image recording | Electron dose: 60 e/Å2 / Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Num. of grids imaged: 1 / Num. of real images: 15771 |
| EM imaging optics | Energyfilter name: GIF Bioquantum / Energyfilter slit width: 20 eV |
| Image scans | Sampling size: 5 µm / Width: 5760 / Height: 4092 |
- Processing
Processing
| EM software |
| |||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTF correction | Details: CTF amplitude correction was performed following motion correction using cryosparc-live Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | |||||||||||||||||||||||||||||||||||||||||||||
| Particle selection | Num. of particles selected: 2639895 | |||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Point symmetry: C2 (2 fold cyclic) | |||||||||||||||||||||||||||||||||||||||||||||
| 3D reconstruction | Resolution: 3.18 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 200061 / Num. of class averages: 1 / Symmetry type: POINT |
 Movie
Movie Controller
Controller






 PDBj
PDBj