[English] 日本語
 Yorodumi
Yorodumi- PDB-6i9r: Large subunit of the human mitochondrial ribosome in complex with... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6i9r | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Large subunit of the human mitochondrial ribosome in complex with Virginiamycin M and Quinupristin | ||||||||||||
 Components Components |
| ||||||||||||
 Keywords Keywords | RIBOSOME / Mitochondria | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationmitochondrial translational termination / mitochondrial translational elongation / translation release factor activity, codon nonspecific / microprocessor complex / Mitochondrial translation elongation / Mitochondrial translation termination / Mitochondrial translation initiation / mitochondrial large ribosomal subunit / peptidyl-tRNA hydrolase / mitochondrial ribosome ...mitochondrial translational termination / mitochondrial translational elongation / translation release factor activity, codon nonspecific / microprocessor complex / Mitochondrial translation elongation / Mitochondrial translation termination / Mitochondrial translation initiation / mitochondrial large ribosomal subunit / peptidyl-tRNA hydrolase / mitochondrial ribosome / Hydrolases; Acting on ester bonds; Endoribonucleases producing 5'-phosphomonoesters / mitochondrial small ribosomal subunit / mitochondrial translation / aminoacyl-tRNA hydrolase activity / anatomical structure morphogenesis / RNA processing / Mitochondrial protein degradation / rescue of stalled ribosome / cellular response to leukemia inhibitory factor / fibrillar center / double-stranded RNA binding / small ribosomal subunit rRNA binding / large ribosomal subunit / cell junction / large ribosomal subunit rRNA binding / 5S rRNA binding / endonuclease activity / mitochondrial inner membrane / negative regulation of translation / nuclear body / rRNA binding / ribosome / structural constituent of ribosome / mitochondrial matrix / translation / ribonucleoprotein complex / protein domain specific binding / mRNA binding / nucleotide binding / synapse / nucleolus / apoptotic process / mitochondrion / RNA binding / nucleoplasm / nucleus / plasma membrane / cytosol Similarity search - Function | ||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human)synthetic construct (others) | ||||||||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 3.9 Å | ||||||||||||
 Authors Authors | Modelska, A. / Aibara, S. / Amunts, A. | ||||||||||||
| Funding support |  Sweden, 1items Sweden, 1items
| ||||||||||||
 Citation Citation |  Journal: Cell Rep / Year: 2021 Journal: Cell Rep / Year: 2021Title: Inhibition of mitochondrial translation suppresses glioblastoma stem cell growth. Authors: Denise Sighel / Michela Notarangelo / Shintaro Aibara / Angela Re / Gianluca Ricci / Marianna Guida / Alessia Soldano / Valentina Adami / Chiara Ambrosini / Francesca Broso / Emanuele ...Authors: Denise Sighel / Michela Notarangelo / Shintaro Aibara / Angela Re / Gianluca Ricci / Marianna Guida / Alessia Soldano / Valentina Adami / Chiara Ambrosini / Francesca Broso / Emanuele Filiberto Rosatti / Sara Longhi / Mariachiara Buccarelli / Quintino G D'Alessandris / Stefano Giannetti / Simone Pacioni / Lucia Ricci-Vitiani / Joanna Rorbach / Roberto Pallini / Sandrine Roulland / Alexey Amunts / Ines Mancini / Angelika Modelska / Alessandro Quattrone /    Abstract: Glioblastoma stem cells (GSCs) resist current glioblastoma (GBM) therapies. GSCs rely highly on oxidative phosphorylation (OXPHOS), whose function requires mitochondrial translation. Here we explore ...Glioblastoma stem cells (GSCs) resist current glioblastoma (GBM) therapies. GSCs rely highly on oxidative phosphorylation (OXPHOS), whose function requires mitochondrial translation. Here we explore the therapeutic potential of targeting mitochondrial translation and report the results of high-content screening with putative blockers of mitochondrial ribosomes. We identify the bacterial antibiotic quinupristin/dalfopristin (Q/D) as an effective suppressor of GSC growth. Q/D also decreases the clonogenicity of GSCs in vitro, consequently dysregulating the cell cycle and inducing apoptosis. Cryoelectron microscopy (cryo-EM) reveals that Q/D binds to the large mitoribosomal subunit, inhibiting mitochondrial protein synthesis and functionally dysregulating OXPHOS complexes. These data suggest that targeting mitochondrial translation could be explored to therapeutically suppress GSC growth in GBM and that Q/D could potentially be repurposed for cancer treatment. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6i9r.cif.gz 6i9r.cif.gz | 2.2 MB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6i9r.ent.gz pdb6i9r.ent.gz | Display |  PDB format PDB format | |
| PDBx/mmJSON format |  6i9r.json.gz 6i9r.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  6i9r_validation.pdf.gz 6i9r_validation.pdf.gz | 1.8 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  6i9r_full_validation.pdf.gz 6i9r_full_validation.pdf.gz | 1.8 MB | Display | |
| Data in XML |  6i9r_validation.xml.gz 6i9r_validation.xml.gz | 220.8 KB | Display | |
| Data in CIF |  6i9r_validation.cif.gz 6i9r_validation.cif.gz | 361.9 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/i9/6i9r https://data.pdbj.org/pub/pdb/validation_reports/i9/6i9r ftp://data.pdbj.org/pub/pdb/validation_reports/i9/6i9r ftp://data.pdbj.org/pub/pdb/validation_reports/i9/6i9r | HTTPS FTP |
-Related structure data
| Related structure data |  4434MC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
+39S ribosomal protein ... , 45 types, 45 molecules 0123456789DEFHIJKLMNOQRSTUVWXY...
-RNA chain , 2 types, 2 molecules AB
| #11: RNA chain | Mass: 499421.250 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Homo sapiens (human) / References: GenBank: 1391841121 Homo sapiens (human) / References: GenBank: 1391841121 |
|---|---|
| #12: RNA chain | Mass: 22022.131 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Homo sapiens (human) / References: GenBank: 1485738021 Homo sapiens (human) / References: GenBank: 1485738021 |
-Protein , 5 types, 5 molecules Pjopq
| #24: Protein | Mass: 20465.348 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Homo sapiens (human) / References: UniProt: A8K9D2 Homo sapiens (human) / References: UniProt: A8K9D2 |
|---|---|
| #44: Protein | Mass: 13696.684 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Homo sapiens (human) / References: UniProt: A8K7J6 Homo sapiens (human) / References: UniProt: A8K7J6 |
| #48: Protein | Mass: 12292.333 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Homo sapiens (human) / References: UniProt: Q9BQC6 Homo sapiens (human) / References: UniProt: Q9BQC6 |
| #49: Protein | Mass: 23674.203 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Homo sapiens (human) / References: UniProt: Q14197, peptidyl-tRNA hydrolase Homo sapiens (human) / References: UniProt: Q14197, peptidyl-tRNA hydrolase |
| #50: Protein | Mass: 25426.895 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Homo sapiens (human) / References: UniProt: Q8TAE8 Homo sapiens (human) / References: UniProt: Q8TAE8 |
-Protein/peptide , 1 types, 1 molecules C
-Non-polymers , 4 types, 139 molecules 

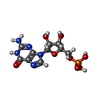




| #54: Chemical | | #55: Chemical | ChemComp-MG / #56: Chemical | ChemComp-G / | #57: Chemical | ChemComp-H8T / | |
|---|
-Details
| Compound details | QUINUPRIST |
|---|
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Large subunit of the human mitochondrial ribosome in complex with Virginiamycin M and Quinupristin Type: RIBOSOME / Entity ID: #1-#52 / Source: NATURAL |
|---|---|
| Molecular weight | Experimental value: NO |
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Buffer solution | pH: 7.5 |
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD |
| Image recording | Electron dose: 38 e/Å2 / Film or detector model: GATAN K2 QUANTUM (4k x 4k) |
- Processing
Processing
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION |
|---|---|
| 3D reconstruction | Resolution: 3.9 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 100000 / Symmetry type: POINT |
| Atomic model building | Protocol: AB INITIO MODEL / Space: REAL |
 Movie
Movie Controller
Controller












 PDBj
PDBj