+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the complex of DNA, Ku70/80, and laXLF. | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | DNA repair / NHEJ / Complex / Lactylation / DNA BINDING PROTEIN / DNA BINDING PROTEIN-DNA complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of ligase activity / DNA ligase IV complex / Ku70:Ku80 complex / negative regulation of t-circle formation / DNA end binding / small-subunit processome assembly / positive regulation of lymphocyte differentiation / DNA-dependent protein kinase complex / DNA-dependent protein kinase-DNA ligase 4 complex / immunoglobulin V(D)J recombination ...positive regulation of ligase activity / DNA ligase IV complex / Ku70:Ku80 complex / negative regulation of t-circle formation / DNA end binding / small-subunit processome assembly / positive regulation of lymphocyte differentiation / DNA-dependent protein kinase complex / DNA-dependent protein kinase-DNA ligase 4 complex / immunoglobulin V(D)J recombination / nonhomologous end joining complex / regulation of smooth muscle cell proliferation / cellular response to X-ray / double-strand break repair via classical nonhomologous end joining / nuclear telomere cap complex / Cytosolic sensors of pathogen-associated DNA / IRF3-mediated induction of type I IFN / recombinational repair / regulation of telomere maintenance / positive regulation of neurogenesis / U3 snoRNA binding / protein localization to chromosome, telomeric region / cellular hyperosmotic salinity response / response to ionizing radiation / 2-LTR circle formation / hematopoietic stem cell proliferation / telomeric DNA binding / positive regulation of protein kinase activity / Lyases; Carbon-oxygen lyases; Other carbon-oxygen lyases / site of DNA damage / T cell differentiation / 5'-deoxyribose-5-phosphate lyase activity / hematopoietic stem cell differentiation / ATP-dependent activity, acting on DNA / telomere maintenance via telomerase / DNA polymerase binding / neurogenesis / activation of innate immune response / DNA helicase activity / telomere maintenance / B cell differentiation / cyclin binding / central nervous system development / cellular response to leukemia inhibitory factor / Nonhomologous End-Joining (NHEJ) / small-subunit processome / enzyme activator activity / protein-DNA complex / cellular response to gamma radiation / double-strand break repair via nonhomologous end joining / Hydrolases; Acting on acid anhydrides; Acting on acid anhydrides to facilitate cellular and subcellular movement / fibrillar center / double-strand break repair / site of double-strand break / double-stranded DNA binding / scaffold protein binding / secretory granule lumen / DNA recombination / transcription regulator complex / ficolin-1-rich granule lumen / damaged DNA binding / chromosome, telomeric region / transcription cis-regulatory region binding / ribonucleoprotein complex / innate immune response / negative regulation of DNA-templated transcription / DNA damage response / ubiquitin protein ligase binding / Neutrophil degranulation / positive regulation of DNA-templated transcription / protein-containing complex binding / nucleolus / positive regulation of transcription by RNA polymerase II / ATP hydrolysis activity / protein-containing complex / DNA binding / RNA binding / extracellular region / nucleoplasm / ATP binding / nucleus / membrane / plasma membrane / cytosol Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.46 Å | |||||||||
 Authors Authors | Liang S | |||||||||
| Funding support |  Hong Kong, 1 items Hong Kong, 1 items
| |||||||||
 Citation Citation |  Journal: Mol Cell / Year: 2025 Journal: Mol Cell / Year: 2025Title: Lactylation of XLF promotes non-homologous end-joining repair and chemoresistance in cancer. Authors: Mingpeng Jin / Bingsong Huang / Xiaoning Yang / Shuyang Wang / Jinhuan Wu / Yiming He / Xin Ding / Xuanhe Wang / Zhe Wang / Jie Yang / Rui Li / Xuan Zhou / Qianwen Wang / Yunhui Li / Lei Li ...Authors: Mingpeng Jin / Bingsong Huang / Xiaoning Yang / Shuyang Wang / Jinhuan Wu / Yiming He / Xin Ding / Xuanhe Wang / Zhe Wang / Jie Yang / Rui Li / Xuan Zhou / Qianwen Wang / Yunhui Li / Lei Li / Wen Zheng / Zhikai Zeng / Chenxi Zhao / Jiaqi Liu / Qian Zhu / Zhihua Kang / Ke Li / Shikang Liang / Yuping Chen / Jian Yuan /  Abstract: Metabolic reprogramming and DNA damage repair are essential in tumorigenesis and chemoresistance, yet their link remains elusive. Here, we show that LDHA deficiency impairs NHEJ and class switch ...Metabolic reprogramming and DNA damage repair are essential in tumorigenesis and chemoresistance, yet their link remains elusive. Here, we show that LDHA deficiency impairs NHEJ and class switch recombination. Additionally, glycolysis-derived lactate promotes XLF lactylation at K288 within its Ku-binding motif (X-KBM) to regulate NHEJ. Mechanistically, DNA damage triggers ATM-mediated GCN5 phosphorylation to increase GCN5-XLF interaction and XLF lactylation, enhancing XLF-Ku80 binding, XLF recruitment to DSBs, and NHEJ efficiency. Cryo-EM structural analysis demonstrates that lactylated X-KBM (laX-KBM) forms a more extensive interface with Ku70/80, inducing conformational changes in the Ku80 vWA domain. XLF lactylation deficiency impairs NHEJ and sensitizes cancer cells to chemotherapy. A specific XLF K288 lactylation peptide inhibitor plus 5-fluorouracil synergistically kills colorectal cancer cells in PDX models with XLF hyperlactylation. These findings highlight that the GCN5-XLF lactylation axis is a critical NHEJ regulator and that targeting XLF lactylation can improve chemotherapy efficiency. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_60744.map.gz emd_60744.map.gz | 26.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-60744-v30.xml emd-60744-v30.xml emd-60744.xml emd-60744.xml | 22.7 KB 22.7 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_60744.png emd_60744.png | 24.7 KB | ||
| Filedesc metadata |  emd-60744.cif.gz emd-60744.cif.gz | 7.1 KB | ||
| Others |  emd_60744_half_map_1.map.gz emd_60744_half_map_1.map.gz emd_60744_half_map_2.map.gz emd_60744_half_map_2.map.gz | 48.9 MB 48.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-60744 http://ftp.pdbj.org/pub/emdb/structures/EMD-60744 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-60744 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-60744 | HTTPS FTP |
-Related structure data
| Related structure data |  9iolMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_60744.map.gz / Format: CCP4 / Size: 52.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_60744.map.gz / Format: CCP4 / Size: 52.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.9557 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_60744_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_60744_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : The complex of DNA, Ku70/80, and laXLF
| Entire | Name: The complex of DNA, Ku70/80, and laXLF |
|---|---|
| Components |
|
-Supramolecule #1: The complex of DNA, Ku70/80, and laXLF
| Supramolecule | Name: The complex of DNA, Ku70/80, and laXLF / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#5 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 168 KDa |
-Macromolecule #1: X-ray repair cross-complementing protein 5
| Macromolecule | Name: X-ray repair cross-complementing protein 5 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO EC number: Hydrolases; Acting on acid anhydrides; Acting on acid anhydrides to facilitate cellular and subcellular movement |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 82.812438 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MVRSGNKAAV VLCMDVGFTM SNSIPGIESP FEQAKKVITM FVQRQVFAEN KDEIALVLFG TDGTDNPLSG GDQYQNITVH RHLMLPDFD LLEDIESKIQ PGSQQADFLD ALIVSMDVIQ HETIGKKFEK RHIEIFTDLS SRFSKSQLDI IIHSLKKCDI S LQFFLPFS ...String: MVRSGNKAAV VLCMDVGFTM SNSIPGIESP FEQAKKVITM FVQRQVFAEN KDEIALVLFG TDGTDNPLSG GDQYQNITVH RHLMLPDFD LLEDIESKIQ PGSQQADFLD ALIVSMDVIQ HETIGKKFEK RHIEIFTDLS SRFSKSQLDI IIHSLKKCDI S LQFFLPFS LGKEDGSGDR GDGPFRLGGH GPSFPLKGIT EQQKEGLEIV KMVMISLEGE DGLDEIYSFS ESLRKLCVFK KI ERHSIHW PCRLTIGSNL SIRIAAYKSI LQERVKKTWT VVDAKTLKKE DIQKETVYCL NDDDETEVLK EDIIQGFRYG SDI VPFSKV DEEQMKYKSE GKCFSVLGFC KSSQVQRRFF MGNQVLKVFA ARDDEAAAVA LSSLIHALDD LDMVAIVRYA YDKR ANPQV GVAFPHIKHN YECLVYVQLP FMEDLRQYMF SSLKNSKKYA PTEAQLNAVD ALIDSMSLAK KDEKTDTLED LFPTT KIPN PRFQRLFQCL LHRALHPREP LPPIQQHIWN MLNPPAEVTT KSQIPLSKIK TLFPLIEAKK KDQVTAQEIF QDNHED GPT AKKLKTEQGG AHFSVSSLAE GSVTSVGSVN PAENFRVLVK QKKASFEEAS NQLINHIEQF LDTNETPYFM KSIDCIR AF REEAIKFSEE QRFNNFLKAL QEKVEIKQLN HFWEIVVQDG ITLITKEEAS GSSVTAEEAK KFLAPKDKPS GDTAAVFE E GGDVDDLLDM I UniProtKB: X-ray repair cross-complementing protein 5 |
-Macromolecule #2: X-ray repair cross-complementing protein 6
| Macromolecule | Name: X-ray repair cross-complementing protein 6 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO EC number: Hydrolases; Acting on acid anhydrides; Acting on acid anhydrides to facilitate cellular and subcellular movement |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 69.945039 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSGWESYYKT EGDEEAEEEQ EENLEASGDY KYSGRDSLIF LVDASKAMFE SQSEDELTPF DMSIQCIQSV YISKIISSDR DLLAVVFYG TEKDKNSVNF KNIYVLQELD NPGAKRILEL DQFKGQQGQK RFQDMMGHGS DYSLSEVLWV CANLFSDVQF K MSHKRIML ...String: MSGWESYYKT EGDEEAEEEQ EENLEASGDY KYSGRDSLIF LVDASKAMFE SQSEDELTPF DMSIQCIQSV YISKIISSDR DLLAVVFYG TEKDKNSVNF KNIYVLQELD NPGAKRILEL DQFKGQQGQK RFQDMMGHGS DYSLSEVLWV CANLFSDVQF K MSHKRIML FTNEDNPHGN DSAKASRART KAGDLRDTGI FLDLMHLKKP GGFDISLFYR DIISIAEDED LRVHFEESSK LE DLLRKVR AKETRKRALS RLKLKLNKDI VISVGIYNLV QKALKPPPIK LYRETNEPVK TKTRTFNTST GGLLLPSDTK RSQ IYGSRQ IILEKEETEE LKRFDDPGLM LMGFKPLVLL KKHHYLRPSL FVYPEESLVI GSSTLFSALL IKCLEKEVAA LCRY TPRRN IPPYFVALVP QEEELDDQKI QVTPPGFQLV FLPFADDKRK MPFTEKIMAT PEQVGKMKAI VEKLRFTYRS DSFEN PVLQ QHFRNLEALA LDLMEPEQAV DLTLPKVEAM NKRLGSLVDE FKELVYPPDY NPEGKVTKRK HDNEGSGSKR PKVEYS EEE LKTHISKGTL GKFTVPMLKE ACRAYGLKSG LKKQELLEAL TKHFQD UniProtKB: X-ray repair cross-complementing protein 6 |
-Macromolecule #5: Peptide from Non-homologous end-joining factor 1
| Macromolecule | Name: Peptide from Non-homologous end-joining factor 1 / type: protein_or_peptide / ID: 5 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 1.536907 KDa |
| Sequence | String: SKVKRKKPRG LFS UniProtKB: Non-homologous end-joining factor 1 |
-Macromolecule #3: DNA (5'-D(P*CP*GP*CP*TP*GP*CP*CP*GP*AP*TP*TP*CP*GP*TP*CP*GP*AP*CP...
| Macromolecule | Name: DNA (5'-D(P*CP*GP*CP*TP*GP*CP*CP*GP*AP*TP*TP*CP*GP*TP*CP*GP*AP*CP*CP*T)-3') type: dna / ID: 3 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 6.969472 KDa |
| Sequence | String: (DC)(DG)(DC)(DT)(DG)(DC)(DC)(DG)(DA)(DT) (DT)(DC)(DG)(DT)(DC)(DG)(DA)(DC)(DC)(DT) (DC)(DG)(DC) |
-Macromolecule #4: DNA (5'-D(P*AP*GP*GP*TP*CP*GP*AP*CP*GP*AP*AP*TP*CP*GP*GP*CP*AP*GP...
| Macromolecule | Name: DNA (5'-D(P*AP*GP*GP*TP*CP*GP*AP*CP*GP*AP*AP*TP*CP*GP*GP*CP*AP*GP*CP*G)-3') type: dna / ID: 4 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 7.156611 KDa |
| Sequence | String: (DG)(DC)(DG)(DA)(DG)(DG)(DT)(DC)(DG)(DA) (DC)(DG)(DA)(DA)(DT)(DC)(DG)(DG)(DC)(DA) (DG)(DC)(DG) |
-Macromolecule #6: INOSITOL HEXAKISPHOSPHATE
| Macromolecule | Name: INOSITOL HEXAKISPHOSPHATE / type: ligand / ID: 6 / Number of copies: 1 / Formula: IHP |
|---|---|
| Molecular weight | Theoretical: 660.035 Da |
| Chemical component information |  ChemComp-IHP: |
-Macromolecule #7: (2S)-2-HYDROXYPROPANOIC ACID
| Macromolecule | Name: (2S)-2-HYDROXYPROPANOIC ACID / type: ligand / ID: 7 / Number of copies: 1 / Formula: 2OP |
|---|---|
| Molecular weight | Theoretical: 90.078 Da |
| Chemical component information | 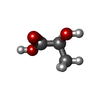 ChemComp-2OP: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.6 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Image recording | Film or detector model: TFS FALCON 4i (4k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller












 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)




































