+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | AtALMT9 with LMNG (cis2 class) | ||||||||||||
 Map data Map data | |||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | Plant / Stomata / Vacuole / ALMT / Ion channel / MEMBRANE PROTEIN | ||||||||||||
| Function / homology | malate transport / Aluminum-activated malate transporter / Aluminium activated malate transporter / plant-type vacuole membrane / monoatomic anion channel activity / Aluminum-activated malate transporter 9 Function and homology information Function and homology information | ||||||||||||
| Biological species |  | ||||||||||||
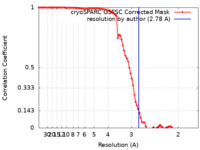
| Method | single particle reconstruction / cryo EM / Resolution: 2.78 Å | ||||||||||||
 Authors Authors | Lee Y / Lee S | ||||||||||||
| Funding support |  Korea, Republic Of, 3 items Korea, Republic Of, 3 items
| ||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2025 Journal: Nat Commun / Year: 2025Title: Structural basis for malate-driven, pore lipid-regulated activation of the Arabidopsis vacuolar anion channel ALMT9. Authors: Yeongmok Lee / Elsa Demes-Causse / Jaemin Yoo / Seo Young Jang / Seoyeon Jung / Justyna Jaślan / Geum-Sook Hwang / Jejoong Yoo / Alexis De Angeli / Sangho Lee /   Abstract: In plant cells, ALMTs are key plasma and vacuolar membrane-localized anion channels regulating plant responses to the environment. Vacuolar ALMTs control anion accumulation in plant cells and, in ...In plant cells, ALMTs are key plasma and vacuolar membrane-localized anion channels regulating plant responses to the environment. Vacuolar ALMTs control anion accumulation in plant cells and, in guard cells, they regulate stomata aperture. The activation of vacuolar ALMTs depends on voltage and cytosolic malate, but the underlying molecular mechanisms remain elusive. Here we report the cryo-EM structures of ALMT9 from Arabidopsis thaliana (AtALMT9), a malate-activated vacuolar anion channel, in plugged and unplugged lipid-bound states. In all these states, membrane lipids interact with the ion conduction pathway of AtALMT9. We identify two unplugged states presenting two distinct pore width profiles. Combining structural and functional analysis we identified conserved residues involved in ion conduction and in the pore lipid interaction. Molecular dynamics simulations revealed a peculiar anion conduction mechanism in AtALMT9. We propose a voltage-dependent activation mechanism based on the competition between pore lipids and malate at the cytosolic entrance of the channel. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_60465.map.gz emd_60465.map.gz | 3.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-60465-v30.xml emd-60465-v30.xml emd-60465.xml emd-60465.xml | 19.7 KB 19.7 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_60465_fsc.xml emd_60465_fsc.xml | 8.4 KB | Display |  FSC data file FSC data file |
| Images |  emd_60465.png emd_60465.png | 59.5 KB | ||
| Filedesc metadata |  emd-60465.cif.gz emd-60465.cif.gz | 6.8 KB | ||
| Others |  emd_60465_half_map_1.map.gz emd_60465_half_map_1.map.gz emd_60465_half_map_2.map.gz emd_60465_half_map_2.map.gz | 59.3 MB 59.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-60465 http://ftp.pdbj.org/pub/emdb/structures/EMD-60465 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-60465 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-60465 | HTTPS FTP |
-Related structure data
| Related structure data |  8ztkMC  8zteC  8ztgC  8zthC  8ztiC  8ztjC  8ztlC  8ztmC  8ztnC  9jtwC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_60465.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_60465.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.858 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_60465_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_60465_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : AtALMT9 with LMNG
| Entire | Name: AtALMT9 with LMNG |
|---|---|
| Components |
|
-Supramolecule #1: AtALMT9 with LMNG
| Supramolecule | Name: AtALMT9 with LMNG / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 130 KDa |
-Macromolecule #1: Aluminum-activated malate transporter 9
| Macromolecule | Name: Aluminum-activated malate transporter 9 / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 67.121008 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MAAKQGSFRH GILEKRERLL SNNGFSDFRF TDIESNDLLE NENCGRRTRL CCCCSCGNLS EKISGVYDDA KDVARKAWEM GVSDPRKIV FSAKIGLALT IVALLIFYQE PNPDLSRYSV WAILTVVVVF EFTIGATLSK GFNRALGTLS AGGLALGMAE L STLFGDWE ...String: MAAKQGSFRH GILEKRERLL SNNGFSDFRF TDIESNDLLE NENCGRRTRL CCCCSCGNLS EKISGVYDDA KDVARKAWEM GVSDPRKIV FSAKIGLALT IVALLIFYQE PNPDLSRYSV WAILTVVVVF EFTIGATLSK GFNRALGTLS AGGLALGMAE L STLFGDWE EIFCTLSIFC IGFLATFMKL YPSMKAYEYG FRVFLLTYCY ILISGFRTGQ FIEVAISRFL LIALGAGVSL GV NMFIYPI WAGEDLHNLV VKNFMNVATS LEGCVNGYLR CLEYERIPSK ILTYQASEDP VYKGYRSAVE STSQEESLMS FAI WEPPHG PYKSFNYPWK NYVKLSGALK HCAFTVMALH GCILSEIQAP EERRQVFRQE LQRVGVEGAK LLRELGEKVK KMEK LGPVD LLFEVHLAAE ELQHKIDKKS YLLVNSECWE IGNRATKESE PQELLSLEDS DPPENHAPPI YAFKSLSEAV LEIPP SWGE KNHREALNHR PTFSKQVSWP ARLVLPPHLE TTNGASPLVE TTKTYESASA LSLATFASLL IEFVARLQNV VDAFKE LSQ KANFKEPEIV TTGTDVEFSG ERVGLGQKIR RCFGM UniProtKB: Aluminum-activated malate transporter 9 |
-Macromolecule #2: 2-(HEXADECANOYLOXY)-1-[(PHOSPHONOOXY)METHYL]ETHYL HEXADECANOATE
| Macromolecule | Name: 2-(HEXADECANOYLOXY)-1-[(PHOSPHONOOXY)METHYL]ETHYL HEXADECANOATE type: ligand / ID: 2 / Number of copies: 2 / Formula: LPP |
|---|---|
| Molecular weight | Theoretical: 648.891 Da |
| Chemical component information |  ChemComp-LPP: |
-Macromolecule #3: water
| Macromolecule | Name: water / type: ligand / ID: 3 / Number of copies: 3 / Formula: HOH |
|---|---|
| Molecular weight | Theoretical: 18.015 Da |
| Chemical component information |  ChemComp-HOH: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1.9 mg/mL | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.6 Component:
| ||||||||||||||||||
| Grid | Model: Au-flat 1.2/1.3 / Material: GOLD / Mesh: 300 / Support film - Material: GOLD / Support film - topology: HOLEY ARRAY / Support film - Film thickness: 45 | ||||||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277.15 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 18 eV |
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 1.9000000000000001 µm / Nominal defocus min: 0.7000000000000001 µm / Nominal magnification: 105000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller













 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)