[English] 日本語
 Yorodumi
Yorodumi- EMDB-44042: Cryo-EM Structure of Sf9 produced recombinant N-acetyltransferase... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM Structure of Sf9 produced recombinant N-acetyltransferase 10 (NAT10) in complex with cytidine-amide-CoA bisubstrate probe and ADP/Mg2+. | ||||||||||||
 Map data Map data | |||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | RNA binding protein / ac4C modification / RNA acetyltransferase | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationtRNA cytidine N4-acetyltransferase activity / rRNA acetylation involved in maturation of SSU-rRNA / 18S rRNA cytidine N-acetyltransferase activity / tRNA acetylation / 90S preribosome / Transferases; Acyltransferases; Transferring groups other than aminoacyl groups / tRNA binding / nucleolus / ATP binding Similarity search - Function | ||||||||||||
| Biological species |  Thermochaetoides thermophila DSM 1495 (fungus) Thermochaetoides thermophila DSM 1495 (fungus) | ||||||||||||
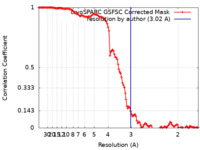
| Method | single particle reconstruction / cryo EM / Resolution: 3.02 Å | ||||||||||||
 Authors Authors | Zhou M / Marmorstein R | ||||||||||||
| Funding support |  United States, 3 items United States, 3 items
| ||||||||||||
 Citation Citation |  Journal: bioRxiv / Year: 2024 Journal: bioRxiv / Year: 2024Title: Molecular Basis for RNA Cytidine Acetylation by NAT10. Authors: Mingyang Zhou / Supuni Thalalla Gamage / Khoa A Tran / David Bartee / Xuepeng Wei / Boyu Yin / Shelley Berger / Jordan L Meier / Ronen Marmorstein Abstract: Human NAT10 acetylates the N4 position of cytidine in RNA, predominantly on rRNA and tRNA, to facilitate ribosome biogenesis and protein translation. NAT10 has been proposed as a therapeutic target ...Human NAT10 acetylates the N4 position of cytidine in RNA, predominantly on rRNA and tRNA, to facilitate ribosome biogenesis and protein translation. NAT10 has been proposed as a therapeutic target in cancers as well as aging-associated pathologies such as Hutchinson-Gilford Progeria Syndrome (HGPS). The ∼120 kDa NAT10 protein uses its acetyl-CoA-dependent acetyltransferase, ATP-dependent helicase, and RNA binding domains in concert to mediate RNA-specific N4-cytidine acetylation. While the biochemical activity of NAT10 is well known, the molecular basis for catalysis of eukaryotic RNA acetylation remains relatively undefined. To provide molecular insights into the RNA-specific acetylation by NAT10, we determined the single particle cryo-EM structures of NAT10 ( NAT10) bound to a bisubstrate cytidine-CoA probe with and without ADP. The structures reveal that NAT10 forms a symmetrical heart-shaped dimer with conserved functional domains surrounding the acetyltransferase active sites harboring the cytidine-CoA probe. Structure-based mutagenesis with analysis of mutants supports the catalytic role of two conserved active site residues (His548 and Tyr549 in NAT10), and two basic patches, both proximal and distal to the active site for RNA-specific acetylation. Yeast complementation analyses and senescence assays in human cells also implicates NAT10 catalytic activity in yeast thermoadaptation and cellular senescence. Comparison of the NAT10 structure to protein lysine and N-terminal acetyltransferase enzymes reveals an unusually open active site suggesting that these enzymes have been evolutionarily tailored for RNA recognition and cytidine-specific acetylation. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_44042.map.gz emd_44042.map.gz | 167.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-44042-v30.xml emd-44042-v30.xml emd-44042.xml emd-44042.xml | 17.8 KB 17.8 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_44042_fsc.xml emd_44042_fsc.xml | 11.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_44042.png emd_44042.png | 109.7 KB | ||
| Filedesc metadata |  emd-44042.cif.gz emd-44042.cif.gz | 6.7 KB | ||
| Others |  emd_44042_half_map_1.map.gz emd_44042_half_map_1.map.gz emd_44042_half_map_2.map.gz emd_44042_half_map_2.map.gz | 165.2 MB 165.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-44042 http://ftp.pdbj.org/pub/emdb/structures/EMD-44042 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-44042 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-44042 | HTTPS FTP |
-Related structure data
| Related structure data |  9b0iMC  9aymC  9b0eC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_44042.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_44042.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.86 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_44042_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_44042_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Sf9 produced recombinant NAT10 in complex with ADP/Mg2+ and chemi...
| Entire | Name: Sf9 produced recombinant NAT10 in complex with ADP/Mg2+ and chemically synthetic cytidine-amide-CoA bisubstrate probe |
|---|---|
| Components |
|
-Supramolecule #1: Sf9 produced recombinant NAT10 in complex with ADP/Mg2+ and chemi...
| Supramolecule | Name: Sf9 produced recombinant NAT10 in complex with ADP/Mg2+ and chemically synthetic cytidine-amide-CoA bisubstrate probe type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Thermochaetoides thermophila DSM 1495 (fungus) Thermochaetoides thermophila DSM 1495 (fungus) |
| Molecular weight | Theoretical: 244 kDa/nm |
-Macromolecule #1: RNA cytidine acetyltransferase
| Macromolecule | Name: RNA cytidine acetyltransferase / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO EC number: Transferases; Acyltransferases; Transferring groups other than aminoacyl groups |
|---|---|
| Source (natural) | Organism:  Thermochaetoides thermophila DSM 1495 (fungus) Thermochaetoides thermophila DSM 1495 (fungus) |
| Molecular weight | Theoretical: 121.475914 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: HHHHHHENLY FQGMTVQKTV DSRIPTLIRN GLQTKKRSFF VVVGDHAKEA IVHLYYIMSS MDVRQNKSVL WAYKKELLGF TSHRKKREA KIKKEIKRGI REPNQADPFE LFISLNDIRY CYYKETDKIL GNTYGMCILQ DFEAITPNIL ARTIETVEGG G LVVLLLKG ...String: HHHHHHENLY FQGMTVQKTV DSRIPTLIRN GLQTKKRSFF VVVGDHAKEA IVHLYYIMSS MDVRQNKSVL WAYKKELLGF TSHRKKREA KIKKEIKRGI REPNQADPFE LFISLNDIRY CYYKETDKIL GNTYGMCILQ DFEAITPNIL ARTIETVEGG G LVVLLLKG MTSLKQLYTM TMDVHARYRT EAHDDVIARF NERFLLSLGS CESCLVIDDE LNVLPISGGK GVKPLPPPDE DE ELSPAAK ELKKIKDELE DTQPIGSLIK LARTVDQAKA LLTFVDAIAE KTLRNTVTLT AARGRGKSAA MGVAIAAAVA YGY SNIFIT SPSPENLKTL FEFVFKGFDA LDYKDHADYT IIQSTNPEFN KAIVRVNIHR NHRQTIQYIR PQDAHVLGQA ELVV IDEAA AIPLPLVKKL MGPYLVFMAS TISGYEGTGR SLSLKLIKQL REQSRAGANP NGGNAVEVDR STLKATKETT SVGGR SLKE ITLSEPIRYA QGDNVEKWLN TLLCLDATLP RSKISTTGCP DPSQCELLHV NRDTLFSFHP VSEKFLQQMV ALYVAS HYK NSPNDLQLMS DAPAHELFVL TGPIQEGRLP EPLCVIQVSL EGKISKQSIL KSLSRGQQPA GDLIPWLVSQ QFQDDEF AS LSGARIVRIA TNPDYMSMGY GSKALQLLVD YYEGKFADLS EDAAAEVPRS IPRVTDAELS KGSLFDDIKV RDMHELPP L FSKLSERRPE KLDYVGVSYG LTQQLHKFWK RAQFVPVYLR QTANDLTGEH TCVMIRPLQD GNDPSWLGAF AADFHKRFL SLLSYKFREF PSILALTIEE SANAGAMLDP SNAPTELTKA ELDQLFTPFD HKRLESYANG LLDYHVVLDL MPTIAQLYFT GRLREAVKL SGLQQAILLA LGLQRKDIDT LATELNLPGS QVLAIFMKIM RKVTQHFGAL VSGAIAAELP DPNKTVGVSK E NAMGIHDD EVVGLKFEAL EQRLEDELDE GGDEALRELR KKQRELIDSL PLDQYEIDGD DDAWKEAEKR VASAAKSGKK VD GTLVSVP SAKAAKRKAE EMAALRDELE KMEKGKERGS KKAKKEKRR UniProtKB: RNA cytidine acetyltransferase |
-Macromolecule #2: ADENOSINE-5'-DIPHOSPHATE
| Macromolecule | Name: ADENOSINE-5'-DIPHOSPHATE / type: ligand / ID: 2 / Number of copies: 2 / Formula: ADP |
|---|---|
| Molecular weight | Theoretical: 427.201 Da |
| Chemical component information |  ChemComp-ADP: |
-Macromolecule #3: MAGNESIUM ION
| Macromolecule | Name: MAGNESIUM ION / type: ligand / ID: 3 / Number of copies: 2 / Formula: MG |
|---|---|
| Molecular weight | Theoretical: 24.305 Da |
-Macromolecule #4: [[(2~{R},3~{S},4~{S},5~{R})-5-(6-aminopurin-9-yl)-4-oxidanyl-3-ph...
| Macromolecule | Name: [[(2~{R},3~{S},4~{S},5~{R})-5-(6-aminopurin-9-yl)-4-oxidanyl-3-phosphonooxy-oxolan-2-yl]methoxy-oxidanyl-phosphoryl] [(3~{R})-4-[[3-[2-[2-[[1-[(2~{R},3~{S},4~{R},5~{R})-5-(hydroxymethyl)-3,4- ...Name: [[(2~{R},3~{S},4~{S},5~{R})-5-(6-aminopurin-9-yl)-4-oxidanyl-3-phosphonooxy-oxolan-2-yl]methoxy-oxidanyl-phosphoryl] [(3~{R})-4-[[3-[2-[2-[[1-[(2~{R},3~{S},4~{R},5~{R})-5-(hydroxymethyl)-3,4-bis(oxidanyl)oxolan-2-yl]-2-oxidanylidene-pyrimidin-4-yl]amino]-2-oxidanylidene-ethyl]sulfanylethylamino]-3-oxidanylidene-propyl]amino]-2,2-dimethyl-3-oxidanyl-4-oxidanylidene-butyl] hydrogen phosphate type: ligand / ID: 4 / Number of copies: 2 / Formula: A1AH4 |
|---|---|
| Molecular weight | Theoretical: 1.050772 KDa |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.8 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 280.15 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 42.9 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 3.0 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model | Chain - Source name: Other / Chain - Initial model type: experimental model Details: The unpublished cryo-EM structure in complex with cytidine-amide-CoA bisubstrate probe and ADP |
|---|---|
| Software | Name:  Coot (ver. 0.9.8.7) Coot (ver. 0.9.8.7) |
| Refinement | Space: REAL / Protocol: RIGID BODY FIT |
| Output model |  PDB-9b0i: |
 Movie
Movie Controller
Controller







 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)