+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Atomic structure of Salmonella SipA/F-actin complex by cryo-EM | |||||||||
 Map data Map data | SipA/F-actin complex | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | actin / Salmonella / type III secretion system / SipA / CELL INVASION | |||||||||
| Function / homology |  Function and homology information Function and homology informationcytoskeletal motor activator activity / myosin heavy chain binding / tropomyosin binding / actin filament bundle / troponin I binding / filamentous actin / mesenchyme migration / skeletal muscle myofibril / actin filament bundle assembly / striated muscle thin filament ...cytoskeletal motor activator activity / myosin heavy chain binding / tropomyosin binding / actin filament bundle / troponin I binding / filamentous actin / mesenchyme migration / skeletal muscle myofibril / actin filament bundle assembly / striated muscle thin filament / skeletal muscle thin filament assembly / actin monomer binding / skeletal muscle fiber development / stress fiber / titin binding / actin filament polymerization / actin filament / filopodium / Hydrolases; Acting on acid anhydrides; Acting on acid anhydrides to facilitate cellular and subcellular movement / calcium-dependent protein binding / lamellipodium / actin binding / cell body / hydrolase activity / protein domain specific binding / calcium ion binding / positive regulation of gene expression / magnesium ion binding / extracellular region / ATP binding / identical protein binding / cytoplasm Similarity search - Function | |||||||||
| Biological species |   Salmonella enterica subsp. enterica serovar Typhimurium str. LT2 (bacteria) Salmonella enterica subsp. enterica serovar Typhimurium str. LT2 (bacteria) | |||||||||
| Method | helical reconstruction / cryo EM / Resolution: 3.2 Å | |||||||||
 Authors Authors | Niedzialkowska E / Runyan L / Kudryashova E / Kudryashov DS / Egelman EH | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Structure / Year: 2024 Journal: Structure / Year: 2024Title: Stabilization of F-actin by Salmonella effector SipA resembles the structural effects of inorganic phosphate and phalloidin. Authors: Ewa Niedzialkowska / Lucas A Runyan / Elena Kudryashova / Edward H Egelman / Dmitri S Kudryashov /  Abstract: Entry of Salmonella into host enterocytes relies on its pathogenicity island 1 effector SipA. We found that SipA binds to F-actin in a 1:2 stoichiometry with sub-nanomolar affinity. A cryo-EM ...Entry of Salmonella into host enterocytes relies on its pathogenicity island 1 effector SipA. We found that SipA binds to F-actin in a 1:2 stoichiometry with sub-nanomolar affinity. A cryo-EM reconstruction revealed that SipA's globular core binds at the groove between actin strands, whereas the extended C-terminal arm penetrates deeply into the inter-strand space, stabilizing F-actin from within. The unusually strong binding of SipA is achieved by a combination of fast association via the core and very slow dissociation dictated by the arm. Similar to P, BeF, and phalloidin, SipA potently inhibited actin depolymerization by actin depolymerizing factor (ADF)/cofilin, which correlated with increased filament stiffness, supporting the hypothesis that F-actin's mechanical properties contribute to the recognition of its nucleotide state by protein partners. The remarkably strong binding to F-actin maximizes the toxin's effects at the injection site while minimizing global influence on the cytoskeleton and preventing pathogen detection by the host cell. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_42161.map.gz emd_42161.map.gz | 204 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-42161-v30.xml emd-42161-v30.xml emd-42161.xml emd-42161.xml | 18.8 KB 18.8 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_42161.png emd_42161.png | 78.7 KB | ||
| Filedesc metadata |  emd-42161.cif.gz emd-42161.cif.gz | 6.7 KB | ||
| Others |  emd_42161_half_map_1.map.gz emd_42161_half_map_1.map.gz emd_42161_half_map_2.map.gz emd_42161_half_map_2.map.gz | 200.6 MB 200.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-42161 http://ftp.pdbj.org/pub/emdb/structures/EMD-42161 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-42161 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-42161 | HTTPS FTP |
-Related structure data
| Related structure data |  8ueeMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_42161.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_42161.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | SipA/F-actin complex | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.08 Å | ||||||||||||||||||||||||||||||||||||
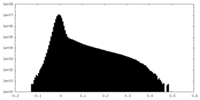
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_42161_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
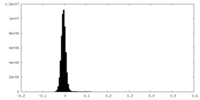
| Density Histograms |
-Half map: #1
| File | emd_42161_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
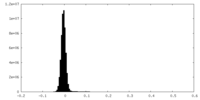
| Density Histograms |
- Sample components
Sample components
-Entire : Salmonella SipA/F-actin complex
| Entire | Name: Salmonella SipA/F-actin complex |
|---|---|
| Components |
|
-Supramolecule #1: Salmonella SipA/F-actin complex
| Supramolecule | Name: Salmonella SipA/F-actin complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:  |
-Supramolecule #2: Actin, alpha skeletal muscle
| Supramolecule | Name: Actin, alpha skeletal muscle / type: complex / ID: 2 / Parent: 1 |
|---|
-Supramolecule #3: SipA
| Supramolecule | Name: SipA / type: complex / ID: 3 / Parent: 1 |
|---|
-Macromolecule #1: Actin, alpha skeletal muscle
| Macromolecule | Name: Actin, alpha skeletal muscle / type: protein_or_peptide / ID: 1 / Number of copies: 7 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 41.875633 KDa |
| Sequence | String: DEDETTALVC DNGSGLVKAG FAGDDAPRAV FPSIVGRPRH QGVMVGMGQK DSYVGDEAQS KRGILTLKYP IE(HIC)GII TNW DDMEKIWHHT FYNELRVAPE EHPTLLTEAP LNPKANREKM TQIMFETFNV PAMYVAIQAV LSLYASGRTT GIVLDSG DG VTHNVPIYEG ...String: DEDETTALVC DNGSGLVKAG FAGDDAPRAV FPSIVGRPRH QGVMVGMGQK DSYVGDEAQS KRGILTLKYP IE(HIC)GII TNW DDMEKIWHHT FYNELRVAPE EHPTLLTEAP LNPKANREKM TQIMFETFNV PAMYVAIQAV LSLYASGRTT GIVLDSG DG VTHNVPIYEG YALPHAIMRL DLAGRDLTDY LMKILTERGY SFVTTAEREI VRDIKEKLCY VALDFENEMA TAASSSSL E KSYELPDGQV ITIGNERFRC PETLFQPSFI GMESAGIHET TYNSIMKCDI DIRKDLYANN VMSGGTTMYP GIADRMQKE ITALAPSTMK IKIIAPPERK YSVWIGGSIL ASLSTFQQMW ITKQEYDEAG PSIVHRKCF UniProtKB: Actin, alpha skeletal muscle |
-Macromolecule #2: Cell invasion protein SipA
| Macromolecule | Name: Cell invasion protein SipA / type: protein_or_peptide / ID: 2 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Salmonella enterica subsp. enterica serovar Typhimurium str. LT2 (bacteria) Salmonella enterica subsp. enterica serovar Typhimurium str. LT2 (bacteria) |
| Molecular weight | Theoretical: 28.413857 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: TGETTSFDEV DGVTSKSIIG KPVQATVHGV DDNKQQSQTA EIVNVKPLAS QLAGVENVKT DTLQSDTTVI TGNKAGTTDN DNSQTDKTG PFSGLKFKQN SFLSTVPSVT NMHSMHFDAR ETFLGVIRKA LEPDTSTPFP VRRAFDGLRA EILPNDTIKS A ALKAQCSD ...String: TGETTSFDEV DGVTSKSIIG KPVQATVHGV DDNKQQSQTA EIVNVKPLAS QLAGVENVKT DTLQSDTTVI TGNKAGTTDN DNSQTDKTG PFSGLKFKQN SFLSTVPSVT NMHSMHFDAR ETFLGVIRKA LEPDTSTPFP VRRAFDGLRA EILPNDTIKS A ALKAQCSD IDKHPELKAK METLKEVITH HPQKEKLAEI ALQFAREAGL TRLKGETDYV LSNVLDGLIG DGSWRAGPAY ES YLNKPGV DRVITTVDGL HMQR UniProtKB: Cell invasion protein SipA |
-Macromolecule #3: ADENOSINE-5'-DIPHOSPHATE
| Macromolecule | Name: ADENOSINE-5'-DIPHOSPHATE / type: ligand / ID: 3 / Number of copies: 7 / Formula: ADP |
|---|---|
| Molecular weight | Theoretical: 427.201 Da |
| Chemical component information |  ChemComp-ADP: |
-Macromolecule #4: MAGNESIUM ION
| Macromolecule | Name: MAGNESIUM ION / type: ligand / ID: 4 / Number of copies: 7 / Formula: MG |
|---|---|
| Molecular weight | Theoretical: 24.305 Da |
-Macromolecule #5: PHOSPHATE ION
| Macromolecule | Name: PHOSPHATE ION / type: ligand / ID: 5 / Number of copies: 7 / Formula: PO4 |
|---|---|
| Molecular weight | Theoretical: 94.971 Da |
| Chemical component information |  ChemComp-PO4: |
-Macromolecule #6: water
| Macromolecule | Name: water / type: ligand / ID: 6 / Number of copies: 1 / Formula: HOH |
|---|---|
| Molecular weight | Theoretical: 18.015 Da |
| Chemical component information |  ChemComp-HOH: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | helical reconstruction |
| Aggregation state | filament |
- Sample preparation
Sample preparation
| Buffer | pH: 8 / Component - Concentration: 25.0 mM / Component - Formula: TRIS-HCl / Component - Name: TRIS Details: Buffer composition: 25 mM TRIS-H-Cl pH 8.0 2 mM DTT |
|---|---|
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 90 % / Chamber temperature: 291 K / Instrument: LEICA EM GP Details: 3 uL of sample was applied on Lacey grid, then sample was blotted for 3 seconds and plunge-froze in liquid ethane. |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number grids imaged: 1 / Number real images: 12452 / Average exposure time: 5.58 sec. / Average electron dose: 48.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.4 µm / Nominal defocus min: 1.2 µm |
| Sample stage | Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Final reconstruction | Applied symmetry - Helical parameters - Δz: 112.075 Å Applied symmetry - Helical parameters - Δ&Phi: 53.656 ° Applied symmetry - Helical parameters - Axial symmetry: C1 (asymmetric) Resolution.type: BY AUTHOR / Resolution: 3.2 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: cryoSPARC (ver. 4.0.2) Details: Reconstruction algorithm: helical refinement and non-uniform refinement in cryoSPARC that is similar to IHRSR algorithm Number images used: 1548993 |
|---|---|
| Startup model | Type of model: PDB ENTRY PDB model - PDB ID: Details: 8D14 model was used for F-actin 1Q5Z model was used for SipA |
| Final angle assignment | Type: NOT APPLICABLE / Software - Name: cryoSPARC (ver. 4.0.2) / Details: Maximum Likelihood |
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: OTHER |
|---|---|
| Output model |  PDB-8uee: |
 Movie
Movie Controller
Controller







 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)