[English] 日本語
 Yorodumi
Yorodumi- EMDB-41438: Cryo-EM structure of HERH-b*01 Fab in complex with HIV-1 Env trim... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of HERH-b*01 Fab in complex with HIV-1 Env trimer BG505.DS SOSIP | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Antibody / Vaccination / VIRAL PROTEIN / IMMUNE SYSTEM / VIRAL PROTEIN-IMMUNE SYSTEM complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationsymbiont-mediated perturbation of host defense response / positive regulation of plasma membrane raft polarization / positive regulation of receptor clustering / host cell endosome membrane / clathrin-dependent endocytosis of virus by host cell / viral protein processing / fusion of virus membrane with host plasma membrane / fusion of virus membrane with host endosome membrane / viral envelope / virion attachment to host cell ...symbiont-mediated perturbation of host defense response / positive regulation of plasma membrane raft polarization / positive regulation of receptor clustering / host cell endosome membrane / clathrin-dependent endocytosis of virus by host cell / viral protein processing / fusion of virus membrane with host plasma membrane / fusion of virus membrane with host endosome membrane / viral envelope / virion attachment to host cell / host cell plasma membrane / virion membrane / structural molecule activity / membrane Similarity search - Function | |||||||||
| Biological species |   Human immunodeficiency virus 1 / Human immunodeficiency virus 1 /  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.39 Å | |||||||||
 Authors Authors | Roark RS / Hoyt F / Hansen B / Fischer E / Shapiro LS / Kwong PD | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Cell / Year: 2024 Journal: Cell / Year: 2024Title: Potent and broad HIV-1 neutralization in fusion peptide-primed SHIV-infected macaques. Authors: Hua Wang / Cheng Cheng / James L Dal Santo / Chen-Hsiang Shen / Tatsiana Bylund / Amy R Henry / Colin A Howe / Juyun Hwang / Nicholas C Morano / Daniel J Morris / Sergei Pletnev / Ryan S ...Authors: Hua Wang / Cheng Cheng / James L Dal Santo / Chen-Hsiang Shen / Tatsiana Bylund / Amy R Henry / Colin A Howe / Juyun Hwang / Nicholas C Morano / Daniel J Morris / Sergei Pletnev / Ryan S Roark / Tongqing Zhou / Bryan T Hansen / Forrest H Hoyt / Timothy S Johnston / Shuyi Wang / Baoshan Zhang / David R Ambrozak / Jordan E Becker / Michael F Bender / Anita Changela / Ridhi Chaudhary / Martin Corcoran / Angela R Corrigan / Kathryn E Foulds / Yicheng Guo / Myungjin Lee / Yingying Li / Bob C Lin / Tracy Liu / Mark K Louder / Marco Mandolesi / Rosemarie D Mason / Krisha McKee / Vinod Nair / Sijy O'Dell / Adam S Olia / Li Ou / Amarendra Pegu / Nagarajan Raju / Reda Rawi / Jesmine Roberts-Torres / Edward K Sarfo / Mallika Sastry / Andrew J Schaub / Stephen D Schmidt / Chaim A Schramm / Cindi L Schwartz / Sarah C Smith / Tyler Stephens / Jonathan Stuckey / I-Ting Teng / John-Paul Todd / Yaroslav Tsybovsky / David J Van Wazer / Shuishu Wang / Nicole A Doria-Rose / Elizabeth R Fischer / Ivelin S Georgiev / Gunilla B Karlsson Hedestam / Zizhang Sheng / Ruth A Woodward / Daniel C Douek / Richard A Koup / Theodore C Pierson / Lawrence Shapiro / George M Shaw / John R Mascola / Peter D Kwong /   Abstract: An antibody-based HIV-1 vaccine will require the induction of potent cross-reactive HIV-1-neutralizing responses. To demonstrate feasibility toward this goal, we combined vaccination targeting the ...An antibody-based HIV-1 vaccine will require the induction of potent cross-reactive HIV-1-neutralizing responses. To demonstrate feasibility toward this goal, we combined vaccination targeting the fusion-peptide site of vulnerability with infection by simian-human immunodeficiency virus (SHIV). In four macaques with vaccine-induced neutralizing responses, SHIV infection boosted plasma neutralization to 45%-77% breadth (geometric mean 50% inhibitory dilution [ID] ∼100) on a 208-strain panel. Molecular dissection of these responses by antibody isolation and cryo-electron microscopy (cryo-EM) structure determination revealed 15 of 16 antibody lineages with cross-clade neutralization to be directed toward the fusion-peptide site of vulnerability. In each macaque, isolated antibodies from memory B cells recapitulated the plasma-neutralizing response, with fusion-peptide-binding antibodies reaching breadths of 40%-60% (50% inhibitory concentration [IC] < 50 μg/mL) and total lineage-concentrations estimates of 50-200 μg/mL. Longitudinal mapping indicated that these responses arose prior to SHIV infection. Collectively, these results provide in vivo molecular examples for one to a few B cell lineages affording potent, broadly neutralizing plasma responses. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_41438.map.gz emd_41438.map.gz | 848.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-41438-v30.xml emd-41438-v30.xml emd-41438.xml emd-41438.xml | 27.1 KB 27.1 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_41438.png emd_41438.png | 115.1 KB | ||
| Filedesc metadata |  emd-41438.cif.gz emd-41438.cif.gz | 7.9 KB | ||
| Others |  emd_41438_half_map_1.map.gz emd_41438_half_map_1.map.gz emd_41438_half_map_2.map.gz emd_41438_half_map_2.map.gz | 1.6 GB 1.6 GB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-41438 http://ftp.pdbj.org/pub/emdb/structures/EMD-41438 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-41438 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-41438 | HTTPS FTP |
-Related structure data
| Related structure data |  8to7MC  8tdxC  8te7C  8tjrC  8tjsC  8tkcC  8tl2C  8tl3C  8tl4C  8tl5C  8tnuC  8to9C  8topC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_41438.map.gz / Format: CCP4 / Size: 1.7 GB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_41438.map.gz / Format: CCP4 / Size: 1.7 GB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.42 Å | ||||||||||||||||||||||||||||||||||||
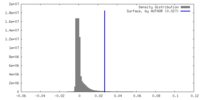
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_41438_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
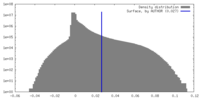
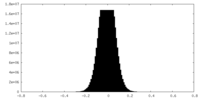
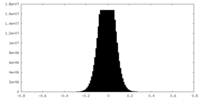
| Density Histograms |
-Half map: #1
| File | emd_41438_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
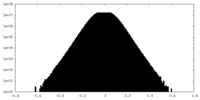
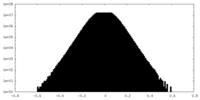
| Density Histograms |
- Sample components
Sample components
-Entire : HERH-b*01 Fab in complex with HIV-1 Env trimer BG505.DS SOSIP
| Entire | Name: HERH-b*01 Fab in complex with HIV-1 Env trimer BG505.DS SOSIP |
|---|---|
| Components |
|
-Supramolecule #1: HERH-b*01 Fab in complex with HIV-1 Env trimer BG505.DS SOSIP
| Supramolecule | Name: HERH-b*01 Fab in complex with HIV-1 Env trimer BG505.DS SOSIP type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#4 |
|---|
-Supramolecule #2: Surface protein gp120, Transmembrane protein gp41
| Supramolecule | Name: Surface protein gp120, Transmembrane protein gp41 / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:   Human immunodeficiency virus 1 Human immunodeficiency virus 1 |
-Supramolecule #3: HERH-b*01 heavy chain, HERH-b*01 light chain
| Supramolecule | Name: HERH-b*01 heavy chain, HERH-b*01 light chain / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #3-#4 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Surface protein gp120
| Macromolecule | Name: Surface protein gp120 / type: protein_or_peptide / ID: 1 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Human immunodeficiency virus 1 Human immunodeficiency virus 1 |
| Molecular weight | Theoretical: 54.086324 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: AENLWVTVYY GVPVWKDAET TLFCASDAKA YETEKHNVWA THACVPTDPN PQEIHLENVT EEFNMWKNNM VEQMHTDIIS LWDQSLKPC VKLTPLCVTL QCTNVTNNIT DDMRGELKNC SFNMTTELRD KKQKVYSLFY RLDVVQINEN QGNRSNNSNK E YRLINCNT ...String: AENLWVTVYY GVPVWKDAET TLFCASDAKA YETEKHNVWA THACVPTDPN PQEIHLENVT EEFNMWKNNM VEQMHTDIIS LWDQSLKPC VKLTPLCVTL QCTNVTNNIT DDMRGELKNC SFNMTTELRD KKQKVYSLFY RLDVVQINEN QGNRSNNSNK E YRLINCNT SACTQACPKV SFEPIPIHYC APAGFAILKC KDKKFNGTGP CPSVSTVQCT HGIKPVVSTQ LLLNGSLAEE EV MIRSENI TNNAKNILVQ FNTPVQINCT RPNNNTRKSI RIGPGQAFYA TGDIIGDIRQ AHCNVSKATW NETLGKVVKQ LRK HFGNNT IIRFANSSGG DLEVTTHSFN CGGEFFYCNT SGLFNSTWIS NTSVQGSNST GSNDSITLPC RIKQIINMWQ RIGQ CMYAP PIQGVIRCVS NITGLILTRD GGSTNSTTET FRPGGGDMRD NWRSELYKYK VVKIEPLGVA PTRCKRRVVG RRRRR R UniProtKB: Envelope glycoprotein gp160 |
-Macromolecule #2: Transmembrane protein gp41
| Macromolecule | Name: Transmembrane protein gp41 / type: protein_or_peptide / ID: 2 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Human immunodeficiency virus 1 Human immunodeficiency virus 1 |
| Molecular weight | Theoretical: 17.146482 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: AVGIGAVFLG FLGAAGSTMG AASMTLTVQA RNLLSGIVQQ QSNLLRAPEA QQHLLKLTVW GIKQLQARVL AVERYLRDQQ LLGIWGCSG KLICCTNVPW NSSWSNRNLS EIWDNMTWLQ WDKEISNYTQ IIYGLLEESQ NQQEKNEQDL LALD UniProtKB: Envelope glycoprotein gp160 |
-Macromolecule #3: HERH-b*01 heavy chain
| Macromolecule | Name: HERH-b*01 heavy chain / type: protein_or_peptide / ID: 3 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 25.558494 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: QVQLQESGPG LVKPSETLSL TCGVSGDSIT RNYYYWHWIR QSPGKGLEGL GYIAYTGGTD YSPSFKSRVT ISRDTSKNQF SLKLTSVTV ADTAVYFCAR ERGDSYIYSY DGVDFWGQGL LVTVSSASTK GPSVFPLAPS SRSTSESTAA LGCLVKDYFP E PVTVSWNS ...String: QVQLQESGPG LVKPSETLSL TCGVSGDSIT RNYYYWHWIR QSPGKGLEGL GYIAYTGGTD YSPSFKSRVT ISRDTSKNQF SLKLTSVTV ADTAVYFCAR ERGDSYIYSY DGVDFWGQGL LVTVSSASTK GPSVFPLAPS SRSTSESTAA LGCLVKDYFP E PVTVSWNS GSLTSGVHTF PAVLQSSGLY SLSSVVTVPS SSLGTQTYVC NVNHKPSNTK VDKRVEIKTC GGLEVLFQGP |
-Macromolecule #4: HERH-b*01 light chain
| Macromolecule | Name: HERH-b*01 light chain / type: protein_or_peptide / ID: 4 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 23.998727 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: DAVLTQSPLS LPITPGEPAS ISCRSSQSLL HTNGETYLNW YQQKTGQRPR LLISQVSKRE IGVPDRFSAS GAGSDFTLKI SRVEAEDVG LYFCGQGLHW PRTFGQGTRV DIKRTVAAPS VFIFPPSEDQ VKSGTVSVVC LLNNFYPREA SVKWKVDGAL K TGNSQESV ...String: DAVLTQSPLS LPITPGEPAS ISCRSSQSLL HTNGETYLNW YQQKTGQRPR LLISQVSKRE IGVPDRFSAS GAGSDFTLKI SRVEAEDVG LYFCGQGLHW PRTFGQGTRV DIKRTVAAPS VFIFPPSEDQ VKSGTVSVVC LLNNFYPREA SVKWKVDGAL K TGNSQESV TEQDSKDNTY SLSSTLTLSS TEYQSHKVYA CEVTHQGLSS PVTKSFNRGE C |
-Macromolecule #8: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 8 / Number of copies: 45 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 / Details: PBS |
|---|---|
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 58.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.8 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)