[English] 日本語
 Yorodumi
Yorodumi- EMDB-40218: CryoEM structure of TnsC(1-503) bound to TnsD(1-318) from E.coli Tn7 -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | CryoEM structure of TnsC(1-503) bound to TnsD(1-318) from E.coli Tn7 | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Transposon / AAA+ ATPase / Oligomer / Complex / DNA BINDING PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationtransposition / DNA recombination / ATP hydrolysis activity / DNA binding / ATP binding Similarity search - Function | |||||||||
| Biological species |  | |||||||||
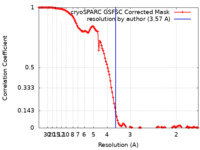
| Method | single particle reconstruction / cryo EM / Resolution: 3.57 Å | |||||||||
 Authors Authors | Shen Y / Guarne A | |||||||||
| Funding support |  Canada, 1 items Canada, 1 items
| |||||||||
 Citation Citation |  Journal: Mol Cell / Year: 2024 Journal: Mol Cell / Year: 2024Title: Assembly of the Tn7 targeting complex by a regulated stepwise process. Authors: Yao Shen / Shreya S Krishnan / Michael T Petassi / Mark A Hancock / Joseph E Peters / Alba Guarné /   Abstract: The Tn7 family of transposons is notable for its highly regulated integration mechanisms, including programmable RNA-guided transposition. The targeting pathways rely on dedicated target selection ...The Tn7 family of transposons is notable for its highly regulated integration mechanisms, including programmable RNA-guided transposition. The targeting pathways rely on dedicated target selection proteins from the TniQ family and the AAA+ adaptor TnsC to recruit and activate the transposase at specific target sites. Here, we report the cryoelectron microscopy (cryo-EM) structures of TnsC bound to the TniQ domain of TnsD from prototypical Tn7 and unveil key regulatory steps stemming from unique behaviors of ATP- versus ADP-bound TnsC. We show that TnsD recruits ADP-bound dimers of TnsC and acts as an exchange factor to release one protomer with exchange to ATP. This loading process explains how TnsC assembles a heptameric ring unidirectionally from the target site. This unique loading process results in functionally distinct TnsC protomers within the ring, providing a checkpoint for target immunity and explaining how insertions at programmed sites precisely occur in a specific orientation across Tn7 elements. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_40218.map.gz emd_40218.map.gz | 158.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-40218-v30.xml emd-40218-v30.xml emd-40218.xml emd-40218.xml | 20.4 KB 20.4 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_40218_fsc.xml emd_40218_fsc.xml | 13.5 KB | Display |  FSC data file FSC data file |
| Images |  emd_40218.png emd_40218.png | 133.4 KB | ||
| Filedesc metadata |  emd-40218.cif.gz emd-40218.cif.gz | 6.4 KB | ||
| Others |  emd_40218_additional_1.map.gz emd_40218_additional_1.map.gz emd_40218_half_map_1.map.gz emd_40218_half_map_1.map.gz emd_40218_half_map_2.map.gz emd_40218_half_map_2.map.gz | 160 MB 165 MB 165 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-40218 http://ftp.pdbj.org/pub/emdb/structures/EMD-40218 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-40218 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-40218 | HTTPS FTP |
-Validation report
| Summary document |  emd_40218_validation.pdf.gz emd_40218_validation.pdf.gz | 783.6 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_40218_full_validation.pdf.gz emd_40218_full_validation.pdf.gz | 783.2 KB | Display | |
| Data in XML |  emd_40218_validation.xml.gz emd_40218_validation.xml.gz | 20.9 KB | Display | |
| Data in CIF |  emd_40218_validation.cif.gz emd_40218_validation.cif.gz | 26.7 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-40218 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-40218 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-40218 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-40218 | HTTPS FTP |
-Related structure data
| Related structure data |  8gluMC  8glwC  8glxC  8vcjC  8vctC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_40218.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_40218.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.855 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: #1
| File | emd_40218_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_40218_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_40218_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Complex of TnsC(1-503) bound to TnsD(1-318) with cofactors ATP and ADP
| Entire | Name: Complex of TnsC(1-503) bound to TnsD(1-318) with cofactors ATP and ADP |
|---|---|
| Components |
|
-Supramolecule #1: Complex of TnsC(1-503) bound to TnsD(1-318) with cofactors ATP and ADP
| Supramolecule | Name: Complex of TnsC(1-503) bound to TnsD(1-318) with cofactors ATP and ADP type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 220 KDa |
-Macromolecule #1: Transposon Tn7 transposition protein TnsC
| Macromolecule | Name: Transposon Tn7 transposition protein TnsC / type: protein_or_peptide / ID: 1 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 59.318926 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGATRIQAVY RDTGVEAYRD NPFIEALPPL QESVNSAASL KSSLQLTSSD LQKSRVIRAH TICRIPDDYF QPLGTHLLLS ERISVMIRG GYVGRNPKTG DLQKHLQNGY ERVQTGELET FRFEEARSTA QSLLLIGCSG SGKTTSLHRI LATYPQVIYH R ELNVEQVV ...String: MGATRIQAVY RDTGVEAYRD NPFIEALPPL QESVNSAASL KSSLQLTSSD LQKSRVIRAH TICRIPDDYF QPLGTHLLLS ERISVMIRG GYVGRNPKTG DLQKHLQNGY ERVQTGELET FRFEEARSTA QSLLLIGCSG SGKTTSLHRI LATYPQVIYH R ELNVEQVV YLKIDCSHNG SLKEICLNFF RALDRALGSN YERRYGLKRH GIETMLALMS QIANAHALGL LVIDEIQHLS RS RSGGSQE MLNFFVTMVN IIGVPVMLIG TPKAREIFEA DLRSARRGAG FGAIFWDPIQ QTQRGKPNQE WIAFTDNLWQ LQL LQRKDA LLSDEVRDVW YELSQGVMDI VVKLFVLAQL RALALGNERI TAGLLRQVYQ DELKPVHPML EALRSGIPER IARY SDLVV PEIDKRLIQL QLDIAAIQEQ TPEEKALQEL DTEDQRHLYL MLKEDYDSSL LIPTIKKAFS QNPTMTRQKL LPLVL QWLM EGETVVSELE KPSKSKKVSP NSSSVDKLAA ALEHHHHHH UniProtKB: Transposon Tn7 transposition protein TnsC |
-Macromolecule #2: Transposon Tn7 transposition protein TnsD
| Macromolecule | Name: Transposon Tn7 transposition protein TnsD / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 36.63602 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MRNFPVPYSN ELIYSTIARA GVYQGIVSPK QLLDEVYGNR KVVATLGLPS HLGVIARHLH QTGRYAVQQL IYEHTLFPLY APFVGKERR DEAIRLMEYQ AQGAVHLMLG VAASRVKSDN RFRYCPDCVA LQLNRYGEAF WQRDWYLPAL PYCPKHGALV F FDRAVDDH ...String: MRNFPVPYSN ELIYSTIARA GVYQGIVSPK QLLDEVYGNR KVVATLGLPS HLGVIARHLH QTGRYAVQQL IYEHTLFPLY APFVGKERR DEAIRLMEYQ AQGAVHLMLG VAASRVKSDN RFRYCPDCVA LQLNRYGEAF WQRDWYLPAL PYCPKHGALV F FDRAVDDH RHQFWALGHT ELLSDYPKDS LSQLTALAAY IAPLLDAPRA QELSPSLEQW TLFYQRLAQD LGLTKSKHIR HD LVAERVR QTFSDEALEK LDLKLAENKD TCWLKSIFRK HRKAFSYLQH SIVWQALLPK LTVIEALQQA SALTEHSITT R UniProtKB: Transposon Tn7 transposition protein TnsD |
-Macromolecule #3: ADENOSINE-5'-TRIPHOSPHATE
| Macromolecule | Name: ADENOSINE-5'-TRIPHOSPHATE / type: ligand / ID: 3 / Number of copies: 2 / Formula: ATP |
|---|---|
| Molecular weight | Theoretical: 507.181 Da |
| Chemical component information |  ChemComp-ATP: |
-Macromolecule #4: MAGNESIUM ION
| Macromolecule | Name: MAGNESIUM ION / type: ligand / ID: 4 / Number of copies: 3 / Formula: MG |
|---|---|
| Molecular weight | Theoretical: 24.305 Da |
-Macromolecule #5: ZINC ION
| Macromolecule | Name: ZINC ION / type: ligand / ID: 5 / Number of copies: 1 / Formula: ZN |
|---|---|
| Molecular weight | Theoretical: 65.409 Da |
-Macromolecule #6: ADENOSINE-5'-DIPHOSPHATE
| Macromolecule | Name: ADENOSINE-5'-DIPHOSPHATE / type: ligand / ID: 6 / Number of copies: 1 / Formula: ADP |
|---|---|
| Molecular weight | Theoretical: 427.201 Da |
| Chemical component information |  ChemComp-ADP: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.4 mg/mL |
|---|---|
| Buffer | pH: 8 Details: 20 mM Tris pH 8.0, 150 mM NaCl, 1.4 mM beta-mercaptoethanol, 5 mM MgCl2 |
| Grid | Model: C-flat-2/1 / Material: COPPER / Mesh: 300 / Pretreatment - Type: GLOW DISCHARGE |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 76.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.75 µm / Nominal defocus min: 1.25 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model |
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Space: REAL / Protocol: OTHER / Overall B value: 208 | ||||||||||||
| Output model |  PDB-8glu: |
 Movie
Movie Controller
Controller







 X (Sec.)
X (Sec.) Y (Row.)
Y (Row.) Z (Col.)
Z (Col.)