[English] 日本語
 Yorodumi
Yorodumi- EMDB-39211: Cryo EM structure of Komagataella phaffii Rat1-Rai1-Rtt103 complex -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo EM structure of Komagataella phaffii Rat1-Rai1-Rtt103 complex | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | transcription termination / RNA polymerase II / TRANSCRIPTION | |||||||||
| Function / homology |  Function and homology information Function and homology informationmRNA 5'-diphosphatase activity / NAD-cap decapping / 5'-3' RNA exonuclease activity / nuclease activity / mRNA 3'-end processing / exonuclease activity / Hydrolases; Acting on acid anhydrides; In phosphorus-containing anhydrides / nuclear-transcribed mRNA catabolic process / RNA polymerase II C-terminal domain binding / DNA-templated transcription termination ...mRNA 5'-diphosphatase activity / NAD-cap decapping / 5'-3' RNA exonuclease activity / nuclease activity / mRNA 3'-end processing / exonuclease activity / Hydrolases; Acting on acid anhydrides; In phosphorus-containing anhydrides / nuclear-transcribed mRNA catabolic process / RNA polymerase II C-terminal domain binding / DNA-templated transcription termination / mRNA processing / rRNA processing / Hydrolases; Acting on ester bonds; Exoribonucleases producing 5'-phosphomonoesters / nucleic acid binding / nucleotide binding / RNA binding / nucleus / metal ion binding / cytosol Similarity search - Function | |||||||||
| Biological species |  Komagataella phaffii (fungus) Komagataella phaffii (fungus) | |||||||||
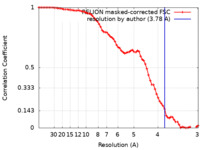
| Method | single particle reconstruction / cryo EM / Resolution: 3.78 Å | |||||||||
 Authors Authors | Yanagisawa T / Murayama Y / Ehara H / Sekine SI | |||||||||
| Funding support |  Japan, 1 items Japan, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: Structural basis of eukaryotic transcription termination by the Rat1 exonuclease complex. Authors: Tatsuo Yanagisawa / Yuko Murayama / Haruhiko Ehara / Mie Goto / Mari Aoki / Shun-Ichi Sekine /  Abstract: The 5´-3´ exoribonuclease Rat1/Xrn2 is responsible for the termination of eukaryotic mRNA transcription by RNAPII. Rat1 forms a complex with its partner proteins, Rai1 and Rtt103, and acts as a ...The 5´-3´ exoribonuclease Rat1/Xrn2 is responsible for the termination of eukaryotic mRNA transcription by RNAPII. Rat1 forms a complex with its partner proteins, Rai1 and Rtt103, and acts as a "torpedo" to bind transcribing RNAPII and dissociate DNA/RNA from it. Here we report the cryo-electron microscopy structures of the Rat1-Rai1-Rtt103 complex and three Rat1-Rai1-associated RNAPII complexes (type-1, type-1b, and type-2) from the yeast, Komagataella phaffii. The Rat1-Rai1-Rtt103 structure revealed that Rat1 and Rai1 form a heterotetramer with a single Rtt103 bound between two Rai1 molecules. In the type-1 complex, Rat1-Rai1 forms a heterodimer and binds to the RNA exit site of RNAPII to extract RNA into the Rat1 exonuclease active site. This interaction changes the RNA path in favor of termination (the "pre-termination" state). The type-1b and type-2 complexes have no bound DNA/RNA, likely representing the "post-termination" states. These structures illustrate the termination mechanism of eukaryotic mRNA transcription. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_39211.map.gz emd_39211.map.gz | 23.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-39211-v30.xml emd-39211-v30.xml emd-39211.xml emd-39211.xml | 18.8 KB 18.8 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_39211_fsc.xml emd_39211_fsc.xml | 7.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_39211.png emd_39211.png | 87.6 KB | ||
| Filedesc metadata |  emd-39211.cif.gz emd-39211.cif.gz | 6.7 KB | ||
| Others |  emd_39211_half_map_1.map.gz emd_39211_half_map_1.map.gz emd_39211_half_map_2.map.gz emd_39211_half_map_2.map.gz | 23.4 MB 23.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-39211 http://ftp.pdbj.org/pub/emdb/structures/EMD-39211 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-39211 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-39211 | HTTPS FTP |
-Validation report
| Summary document |  emd_39211_validation.pdf.gz emd_39211_validation.pdf.gz | 878.2 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_39211_full_validation.pdf.gz emd_39211_full_validation.pdf.gz | 877.7 KB | Display | |
| Data in XML |  emd_39211_validation.xml.gz emd_39211_validation.xml.gz | 12.7 KB | Display | |
| Data in CIF |  emd_39211_validation.cif.gz emd_39211_validation.cif.gz | 17.4 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-39211 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-39211 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-39211 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-39211 | HTTPS FTP |
-Related structure data
| Related structure data |  8yf5MC  8yfeC  8yfqC  8yfrC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_39211.map.gz / Format: CCP4 / Size: 30.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_39211.map.gz / Format: CCP4 / Size: 30.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.494 Å | ||||||||||||||||||||||||||||||||||||
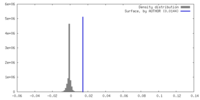
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_39211_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
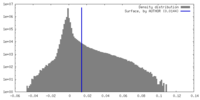
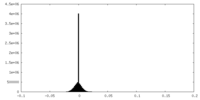
| Density Histograms |
-Half map: #1
| File | emd_39211_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
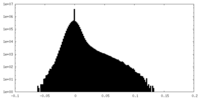
| Density Histograms |
- Sample components
Sample components
-Entire : Komagataella phaffii Rat1-Rai1-Rtt103 complex
| Entire | Name: Komagataella phaffii Rat1-Rai1-Rtt103 complex |
|---|---|
| Components |
|
-Supramolecule #1: Komagataella phaffii Rat1-Rai1-Rtt103 complex
| Supramolecule | Name: Komagataella phaffii Rat1-Rai1-Rtt103 complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Komagataella phaffii (fungus) Komagataella phaffii (fungus) |
-Macromolecule #1: 5'-3' exoribonuclease
| Macromolecule | Name: 5'-3' exoribonuclease / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO EC number: Hydrolases; Acting on ester bonds; Exoribonucleases producing 5'-phosphomonoesters |
|---|---|
| Source (natural) | Organism:  Komagataella phaffii (fungus) Komagataella phaffii (fungus) |
| Molecular weight | Theoretical: 115.314461 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGVPALFRWL SRKYPKIISP VIQDEDVDID GESRPTRYED PNPNGELDNL YLDMNGIVHP CSHPEHKPVP ETEDEMMLDV FAYTENVIM MARPRKVIYI AVDGVAPRAK MNQQRSRRFR SAQDAKDANE KKAAELKEME KKGEIIDDAI KNKKTWDSNA I TPGTPFMH ...String: MGVPALFRWL SRKYPKIISP VIQDEDVDID GESRPTRYED PNPNGELDNL YLDMNGIVHP CSHPEHKPVP ETEDEMMLDV FAYTENVIM MARPRKVIYI AVDGVAPRAK MNQQRSRRFR SAQDAKDANE KKAAELKEME KKGEIIDDAI KNKKTWDSNA I TPGTPFMH RLADSLRYWA AYKLTTDPGW SGIEVIISDA SVPGEGEHKI MSYVRSLRSS PKHDPNTTHC IYGLAAALIF LG LATHEPH FKILREDVFA QDKKSYSLQD QLRMTDIERQ ELKDKKTPFL WLHLNILREY LQIELNVPGL SFPFDLEKSI DDW VFICFF CGNDFLPHLP SLDVRDNSIT TLVTIWKQIL PTMKGYLTTD GYLNLPAVER LLAELAKKED YIFRKRYEDE KRSL ENQKR RKLAQEQSSA RSQNAPNIST GKDKAPLTPN QNIPLYTTSG ESVGIKMTDS EMVNNSALIT KANEANKSIA ELLKQ NLQN EINKKRKISN EEQEVVKESV EEVVEEEDDV LVTSDPEDSS TEILIPKNEE IRLWEPGYRK RYYETKFHTK DPQKVK KIA RNMVQKYIEG VSWVLLYYYQ GCPSWNWYYP YHYAPFAADF VNLSELKIEF VEGTPFRPYE QLMSVLPAAS SHNLPDV FR SLMSDANSEI IDFYPEEFPL DMNGKKVIWQ AIPLLPFIDE NRLLKAVQSK YDQLTEDEKF RNTNRSEILV LGRSHSHY P TLVKELYEEG KDSYEFQVDS SGVSGVAIKL QSFDRSGVLR LPVKQLEGYR HYPDISNRDF LMVEFKQLPK SHAKSMILS GLIPHLRRLT QEDKDSILYG GTNFYGRNRF SPEENADFKQ YIGPHGKSQY LPRQGGYKAF IQIHSDEAKG HRHGIYHGGS HTETEFRRG GGYHQHGNRG GRGGYQGNQG YQANSGGYQN SYQGSYQGGY RGGYQGGSQG RYQAGYQSGY QGGYQGEYKN G YQGGYQGN QGNQGYNRQT YNASKSGTLP MKRRHNSGPS SGLEVLFQ UniProtKB: 5'-3' exoribonuclease |
-Macromolecule #2: Decapping nuclease
| Macromolecule | Name: Decapping nuclease / type: protein_or_peptide / ID: 2 / Number of copies: 2 / Enantiomer: LEVO EC number: Hydrolases; Acting on acid anhydrides; In phosphorus-containing anhydrides |
|---|---|
| Source (natural) | Organism:  Komagataella phaffii (fungus) Komagataella phaffii (fungus) |
| Molecular weight | Theoretical: 44.595996 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: GPGMSKEKIL PLAARSKKAM LRQPKQVAYF SRDLNYKTHP DRSNLSYYYL PDGDIDNSID LSVGSKHFLL GDSVELSKLD PILLALKEI EKESGAKTKD RIITWRGIMR KLLTLPYDSE EDFVLDVVSF DGQLFIQFNV PYLKSKDVQK QGDTEFHKKL Q FSGYKFEK ...String: GPGMSKEKIL PLAARSKKAM LRQPKQVAYF SRDLNYKTHP DRSNLSYYYL PDGDIDNSID LSVGSKHFLL GDSVELSKLD PILLALKEI EKESGAKTKD RIITWRGIMR KLLTLPYDSE EDFVLDVVSF DGQLFIQFNV PYLKSKDVQK QGDTEFHKKL Q FSGYKFEK MATLPKPWPE CTRKEIDSRA KSKCNNIEQY GAIVRTGISR IKILIGGAVA CTADYYDEND PLSRYIELKT TR TINQYKD MIAFEKKLFR TWAQCFLLGI PKIIYGFRDD NCILRTVEEF STNDIPLMVK NNPLNEQPKK ENCYMSSINF YGA VVEWLN ESVKDDQVWK LSYAKRNRQY LVLKEVTDEN EKQQIVDSAI PAWFKEWRSE LRNSEGNI UniProtKB: Decapping nuclease |
-Macromolecule #3: Exonuclease Rat1p and Rai1p interacting protein
| Macromolecule | Name: Exonuclease Rat1p and Rai1p interacting protein / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Komagataella phaffii (fungus) Komagataella phaffii (fungus) |
| Molecular weight | Theoretical: 42.27293 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSYSKDIVLE KLASLEETQI SIQSIGQWCL FHHRHAPETV AIWSEFVGST QTKKLAGLYL ANEIIQQSRA KRKTTFLDEF AKVLPSTLE QIYPSMISTH QAKLKRIIDV WSQRKIFDSN LIHRLYQSIQ DQKAYNGLSS SSSNSPPINS GDLAPEISSL S TLFNKIST ...String: MSYSKDIVLE KLASLEETQI SIQSIGQWCL FHHRHAPETV AIWSEFVGST QTKKLAGLYL ANEIIQQSRA KRKTTFLDEF AKVLPSTLE QIYPSMISTH QAKLKRIIDV WSQRKIFDSN LIHRLYQSIQ DQKAYNGLSS SSSNSPPINS GDLAPEISSL S TLFNKIST LKSSTSLVVN QINEQYSSLF DSETLPGTDI YLKQLGDLST LITSARSKSQ ETQELRESII NELKKLIQVQ ES WITKDAE SSGSLDEKLA TVQQKESELK EFINDVEEED GVPQYAASSD EEGDENVSKK RKMESPPTET VDSGEQPTNP VHP QLSSIL ESLSRTTGLT PEPASTSDES ATATQKQDET PVSSSINPAL ASLLSKLNGL EVLFQ UniProtKB: Exonuclease Rat1p and Rai1p interacting protein |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 Component:
| ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 300 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 90 sec. | ||||||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 75 % / Chamber temperature: 283 K / Instrument: LEICA EM GP / Details: EMGP2. |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 61.9 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.4 µm / Nominal defocus min: 1.6 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller







 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)