+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | SARS-CoV-2 Omicron BQ.1.1 RBD complexed with human ACE2 | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | RBD / VIRAL PROTEIN / VIRUS | |||||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of amino acid transport / angiotensin-converting enzyme 2 / positive regulation of L-proline import across plasma membrane / Hydrolases; Acting on peptide bonds (peptidases); Metallocarboxypeptidases / angiotensin-mediated drinking behavior / viral translation / positive regulation of gap junction assembly / regulation of systemic arterial blood pressure by renin-angiotensin / tryptophan transport / regulation of cardiac conduction ...positive regulation of amino acid transport / angiotensin-converting enzyme 2 / positive regulation of L-proline import across plasma membrane / Hydrolases; Acting on peptide bonds (peptidases); Metallocarboxypeptidases / angiotensin-mediated drinking behavior / viral translation / positive regulation of gap junction assembly / regulation of systemic arterial blood pressure by renin-angiotensin / tryptophan transport / regulation of cardiac conduction / maternal process involved in female pregnancy / peptidyl-dipeptidase activity / regulation of vasoconstriction / transporter activator activity / Metabolism of Angiotensinogen to Angiotensins / carboxypeptidase activity / angiotensin maturation / viral life cycle / Attachment and Entry / receptor-mediated endocytosis of virus by host cell / metallocarboxypeptidase activity / positive regulation of cardiac muscle contraction / regulation of cytokine production / blood vessel diameter maintenance / negative regulation of smooth muscle cell proliferation / brush border membrane / negative regulation of ERK1 and ERK2 cascade / positive regulation of reactive oxygen species metabolic process / metallopeptidase activity / endocytic vesicle membrane / regulation of cell population proliferation / virus receptor activity / regulation of inflammatory response / endopeptidase activity / symbiont-mediated disruption of host tissue / Maturation of spike protein / Translation of Structural Proteins / Virion Assembly and Release / host cell surface / host extracellular space / symbiont-mediated-mediated suppression of host tetherin activity / Induction of Cell-Cell Fusion / Potential therapeutics for SARS / structural constituent of virion / membrane fusion / Attachment and Entry / entry receptor-mediated virion attachment to host cell / host cell endoplasmic reticulum-Golgi intermediate compartment membrane / positive regulation of viral entry into host cell / receptor-mediated virion attachment to host cell / apical plasma membrane / host cell surface receptor binding / cilium / symbiont-mediated suppression of host innate immune response / membrane raft / endocytosis involved in viral entry into host cell / endoplasmic reticulum lumen / receptor ligand activity / fusion of virus membrane with host plasma membrane / fusion of virus membrane with host endosome membrane / viral envelope / symbiont entry into host cell / virion attachment to host cell / host cell plasma membrane / SARS-CoV-2 activates/modulates innate and adaptive immune responses / virion membrane / cell surface / negative regulation of transcription by RNA polymerase II / extracellular space / extracellular exosome / extracellular region / zinc ion binding / identical protein binding / membrane / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.69 Å | |||||||||
 Authors Authors | Li W / Xie Y | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: EMBO J / Year: 2024 Journal: EMBO J / Year: 2024Title: Key mechanistic features of the trade-off between antibody escape and host cell binding in the SARS-CoV-2 Omicron variant spike proteins. Authors: Weiwei Li / Zepeng Xu / Tianhui Niu / Yufeng Xie / Zhennan Zhao / Dedong Li / Qingwen He / Wenqiao Sun / Kaiyuan Shi / Wenjing Guo / Zhen Chang / Kefang Liu / Zheng Fan / Jianxun Qi / George F Gao /  Abstract: Since SARS-CoV-2 Omicron variant emerged, it is constantly evolving into multiple sub-variants, including BF.7, BQ.1, BQ.1.1, XBB, XBB.1.5 and the recently emerged BA.2.86 and JN.1. Receptor binding ...Since SARS-CoV-2 Omicron variant emerged, it is constantly evolving into multiple sub-variants, including BF.7, BQ.1, BQ.1.1, XBB, XBB.1.5 and the recently emerged BA.2.86 and JN.1. Receptor binding and immune evasion are recognized as two major drivers for evolution of the receptor binding domain (RBD) of the SARS-CoV-2 spike (S) protein. However, the underlying mechanism of interplay between two factors remains incompletely understood. Herein, we determined the structures of human ACE2 complexed with BF.7, BQ.1, BQ.1.1, XBB and XBB.1.5 RBDs. Based on the ACE2/RBD structures of these sub-variants and a comparison with the known complex structures, we found that R346T substitution in the RBD enhanced ACE2 binding upon an interaction with the residue R493, but not Q493, via a mechanism involving long-range conformation changes. Furthermore, we found that R493Q and F486V exert a balanced impact, through which immune evasion capability was somewhat compromised to achieve an optimal receptor binding. We propose a "two-steps-forward and one-step-backward" model to describe such a compromise between receptor binding affinity and immune evasion during RBD evolution of Omicron sub-variants. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_37467.map.gz emd_37467.map.gz | 56.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-37467-v30.xml emd-37467-v30.xml emd-37467.xml emd-37467.xml | 19.7 KB 19.7 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_37467.png emd_37467.png | 49.1 KB | ||
| Filedesc metadata |  emd-37467.cif.gz emd-37467.cif.gz | 6.9 KB | ||
| Others |  emd_37467_half_map_1.map.gz emd_37467_half_map_1.map.gz emd_37467_half_map_2.map.gz emd_37467_half_map_2.map.gz | 59.5 MB 59.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-37467 http://ftp.pdbj.org/pub/emdb/structures/EMD-37467 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-37467 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-37467 | HTTPS FTP |
-Related structure data
| Related structure data |  8wdyMC  8wdrC  8wdsC  8wdzC  8we0C  8we1C  8we4C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_37467.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_37467.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.85 Å | ||||||||||||||||||||||||||||||||||||
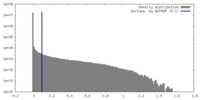
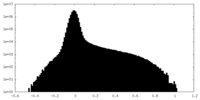
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_37467_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
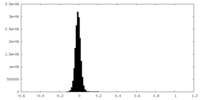
| Density Histograms |
-Half map: #1
| File | emd_37467_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
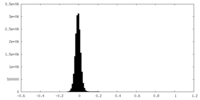
| Density Histograms |
- Sample components
Sample components
-Entire : SARS-CoV-2 Omicron BQ.1.1 RBD complexed with human ACE2
| Entire | Name: SARS-CoV-2 Omicron BQ.1.1 RBD complexed with human ACE2 |
|---|---|
| Components |
|
-Supramolecule #1: SARS-CoV-2 Omicron BQ.1.1 RBD complexed with human ACE2
| Supramolecule | Name: SARS-CoV-2 Omicron BQ.1.1 RBD complexed with human ACE2 type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|
-Supramolecule #2: ACE2
| Supramolecule | Name: ACE2 / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Supramolecule #3: BQ.1.1 RBD
| Supramolecule | Name: BQ.1.1 RBD / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #2 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Angiotensin-converting enzyme 2
| Macromolecule | Name: Angiotensin-converting enzyme 2 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO / EC number: angiotensin-converting enzyme 2 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 92.556695 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MSSSSWLLLS LVAVTAAQST IEEQAKTFLD KFNHEAEDLF YQSSLASWNY NTNITEENVQ NMNNAGDKWS AFLKEQSTLA QMYPLQEIQ NLTVKLQLQA LQQNGSSVLS EDKSKRLNTI LNTMSTIYST GKVCNPDNPQ ECLLLEPGLN EIMANSLDYN E RLWAWESW ...String: MSSSSWLLLS LVAVTAAQST IEEQAKTFLD KFNHEAEDLF YQSSLASWNY NTNITEENVQ NMNNAGDKWS AFLKEQSTLA QMYPLQEIQ NLTVKLQLQA LQQNGSSVLS EDKSKRLNTI LNTMSTIYST GKVCNPDNPQ ECLLLEPGLN EIMANSLDYN E RLWAWESW RSEVGKQLRP LYEEYVVLKN EMARANHYED YGDYWRGDYE VNGVDGYDYS RGQLIEDVEH TFEEIKPLYE HL HAYVRAK LMNAYPSYIS PIGCLPAHLL GDMWGRFWTN LYSLTVPFGQ KPNIDVTDAM VDQAWDAQRI FKEAEKFFVS VGL PNMTQG FWENSMLTDP GNVQKAVCHP TAWDLGKGDF RILMCTKVTM DDFLTAHHEM GHIQYDMAYA AQPFLLRNGA NEGF HEAVG EIMSLSAATP KHLKSIGLLS PDFQEDNETE INFLLKQALT IVGTLPFTYM LEKWRWMVFK GEIPKDQWMK KWWEM KREI VGVVEPVPHD ETYCDPASLF HVSNDYSFIR YYTRTLYQFQ FQEALCQAAK HEGPLHKCDI SNSTEAGQKL FNMLRL GKS EPWTLALENV VGAKNMNVRP LLNYFEPLFT WLKDQNKNSF VGWSTDWSPY ADQSIKVRIS LKSALGDKAY EWNDNEM YL FRSSVAYAMR QYFLKVKNQM ILFGEEDVRV ANLKPRISFN FFVTAPKNVS DIIPRTEVEK AIRMSRSRIN DAFRLNDN S LEFLGIQPTL GPPNQPPVSI WLIVFGVVMG VIVVGIVILI FTGIRDRKKK NKARSGENPY ASIDISKGEN NPGFQNTDD VQTSF UniProtKB: Angiotensin-converting enzyme 2 |
-Macromolecule #2: Spike protein S1
| Macromolecule | Name: Spike protein S1 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 25.186553 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: RVQPTESIVR FPNITNLCPF DEVFNATTFA SVYAWNRKRI SNCVADYSVL YNFAPFFAFK CYGVSPTKLN DLCFTNVYAD SFVIRGNEV SQIAPGQTGN IADYNYKLPD DFTGCVIAWN SNKLDSTVGG NYNYRYRLFR KSKLKPFERD ISTEIYQAGN K PCNGVAGV ...String: RVQPTESIVR FPNITNLCPF DEVFNATTFA SVYAWNRKRI SNCVADYSVL YNFAPFFAFK CYGVSPTKLN DLCFTNVYAD SFVIRGNEV SQIAPGQTGN IADYNYKLPD DFTGCVIAWN SNKLDSTVGG NYNYRYRLFR KSKLKPFERD ISTEIYQAGN K PCNGVAGV NCYFPLQSYG FRPTYGVGHQ PYRVVVLSFE LLHAPATVCG PKKSTNLVKN KCVNF UniProtKB: Spike glycoprotein |
-Macromolecule #5: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 5 / Number of copies: 5 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Macromolecule #6: ZINC ION
| Macromolecule | Name: ZINC ION / type: ligand / ID: 6 / Number of copies: 1 / Formula: ZN |
|---|---|
| Molecular weight | Theoretical: 65.409 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.2 mg/mL |
|---|---|
| Buffer | pH: 8 |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller













 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)